Chemistry Chapter 3 Study Guide 1 27 Flashcards Learn Test Match Q Chat Created by kaelin hughes Terms in this set 27 actinide the 14 elements with atomic numbers from 90 to 103 radioactive but none beyond 92 metal elements with luster high melting points bend without breaking and are great conductors of heat and electricity metalloid
CHAPTER 3 STUDY GUIDE Matter Properties and Changes Section 3 1 Properties of Matter In your textbook read about physical properties and chemical properties of matter Use each of the terms below just once to complete the passage chemical mass physical density properties substance Matter is anything with 1 and volume A Monoatomic ion an ion formed from only one atom polyatomic ion an ion made of two or more atoms Roman Numeral positive charge molecular compounds 2 or more nonmetals ionic compounds
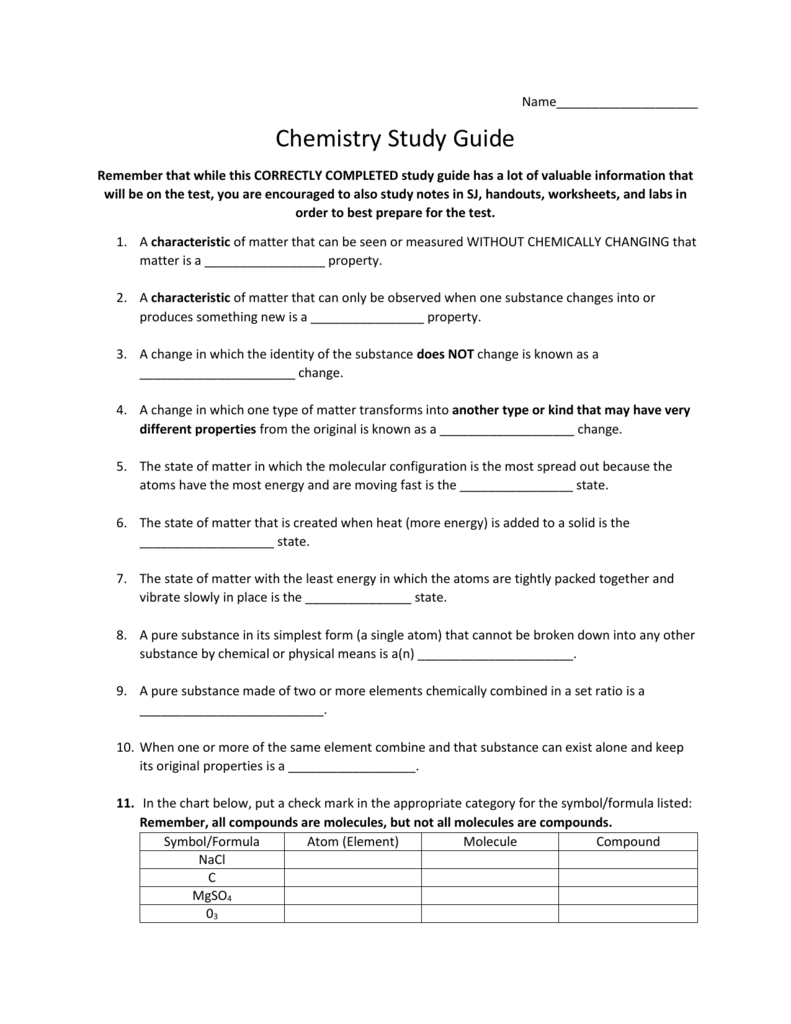
Chemistry Chapter 3 Study Guide
 Chemistry Chapter 3 Study Guide
Chemistry Chapter 3 Study Guide
https://online.fliphtml5.com/trvn/wjvh/files/large/3.jpg
Chapter 3 Solutions by Student Study Guide for Chemistry 11th Edition Edit edition 91 688 ratings for this chapter s solutions Solutions for Chapter 3 Get solutions Looking for the textbook We have solutions for your book This problem has been solved Problem 1P Chapter CH3 Problem 1P Step by step solution Step 1 of 3
Templates are pre-designed files or files that can be utilized for numerous functions. They can save time and effort by offering a ready-made format and design for developing various kinds of content. Templates can be used for personal or expert jobs, such as resumes, invitations, leaflets, newsletters, reports, presentations, and more.
Chemistry Chapter 3 Study Guide

Solutions Study Guide For Content Mastery Bomexotic

Solutions Short Notes Solution Chapter In Chemistry Class 12 Notes

Chemistry 1 Chapter 3 Study Guide Packet Matter Chemical Substances

AP CHEMISTRY STUDY GUIDE eBook Rental Ap Chemistry Chemistry Study
Chemistry Study Guide

Modern Chemistry Chapter 1 Test

http://www.mchsapchemistry.com/uploads/7/7/1/5/7715124/ch_3_study_guide_key.pdf
CHAPTER 3 REVIEW Atoms The Building Blocks of Matter SECTION 3 SHORT ANSWER Answer the following questions in the space provided 1 Explain the difference between the mass numberand the atomic numberof a nuclide Mass number is the total number of protons and neutrons in the nucleus of an isotope

https://quizlet.com/332700590/introduction-to-chemistry-chapter-3-study-guide-flash-cards/
Introduction to Chemistry Chapter 3 Study Guide Essay 1 What contribution s did Dmitri Mendeleev make to chemistry Click the card to flip He organized the first periodic table according to atomic mass He was also able to predict the properties of undiscovered elements Click the card to flip 1 17 Flashcards Test Match Q Chat Created by
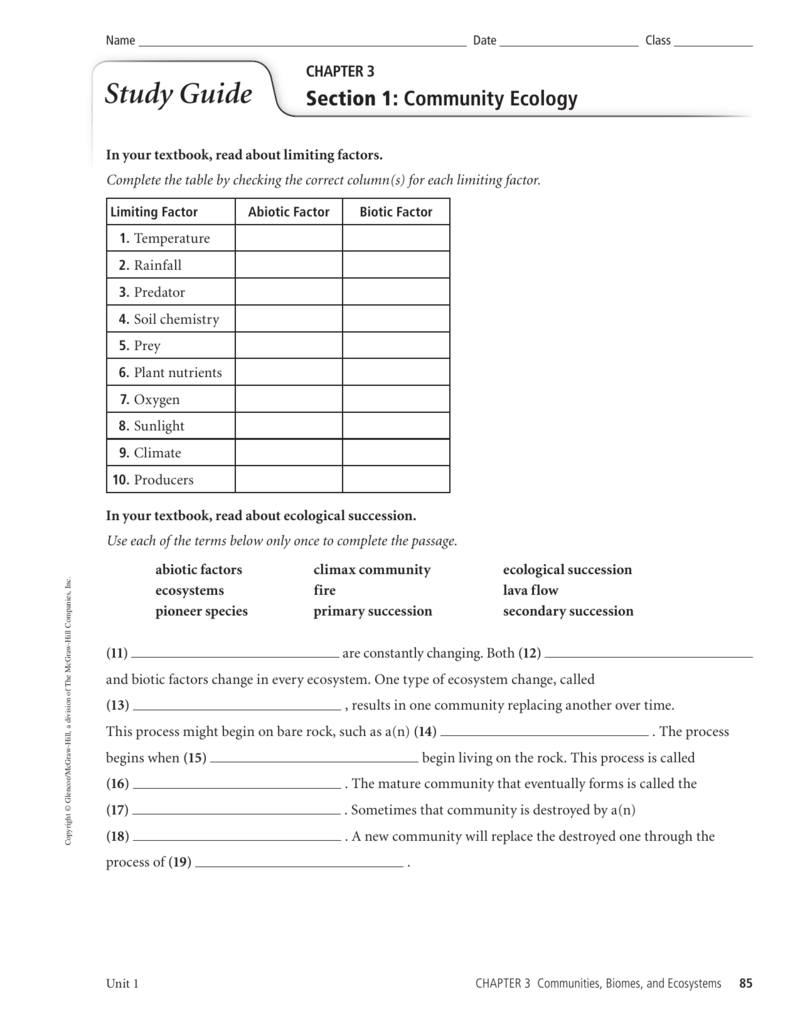
https://glencoe.mheducation.com/sites/007874637x/student_view0/chapter3/
Careers in Chemistry Concepts in Motion Interactive Tutor Personal Tutor Vocabulary eFlashcards Section 1 Properties of Matter Section 2 Changes in Matter Section 3 Mixtures of Matter Section 4 Elements and Compounds

https://quizlet.com/615035178/chemistry-chapter-3-study-guide-flash-cards/
Chemists have made some elements that do not exist naturally on the periodic table TRUE The elements on the current periodic table are placed in order of increasing Atomic Number The alkali metals are in what group 1A

https://quizlet.com/1593864/chapter-3-chemistry-study-guide-flash-cards/
Test Match Q Chat Created by wolfluvinfreak Terms in this set 28 unchanging composition physical or chemical characteristics of a substance physical property a characteristic that can be observed or measured without chanign the sample s composition ex shape color weight density chemical property
32 Study Guide for An Introduction to Chemistry Exercise 3 3 Cations and Anions Identify each of the following as a cation or an anion and determine the charge on each Obj 22 a magnesium atom with 12 protons and 10 electrons 12 10 2 This is a 2 cation b fluorine atom with 9 protons and 10 electrons Central science electrons and structure of atoms bonding and interactions reactions kinetic theory the mole and quantifying matter matter and energy and carbon chemistry dont have to know all of them what are the big ideas of chemistry often linked what is the relationship between pure and applied chemistry
Accelerated Chemistry 3 Atoms The Building Blocks of Matter CHAPTER 3 STUDY GUIDE Atoms The Building Blocks of Matter SECTION 3 1 The Atom from Philosophical Idea to Scientific Theory Pages 67 71 SHORT ANSWER Answer the following questions in the space provided 1 Why is Democritus s view of matter considered only an idea while