Medical Device Quality Systems Manual A Small Entity Compliance Guide Imprint CRC Press Pages 52 eBook ISBN 9780429130410 ABSTRACT Andrew Lowery Judy Strojny and Joseph Puleo Division of Small Manufacturers Assistance Office of Health and Industry Programs CENTER FOR DEVICES AND RADIOLOGICAL HEALTH CDRH December 1996 3 Design Controls Introduction
Medical Device Quality Systems Manual A Small Entity Compliance Guide The FDA Worldwide Quality System Requirements Guidebook for Medical Devices and other device specific guidance Covers the Quality System regulation and the basic Good Manufacturing Practices GMP requirements that all manufacturers and distributors must consider when they plan to manufacture medical devices including medical device kits trays or packs for distribution in the United States
Medical Device Quality Systems Manual A Small Entity Compliance Guide
 Medical Device Quality Systems Manual A Small Entity Compliance Guide
Medical Device Quality Systems Manual A Small Entity Compliance Guide
https://i.ytimg.com/vi/_3iCvrVJB0E/maxresdefault.jpg
This manual covers the Quality System regulation and the basic Good Manufacturing Practices GMP requirements that all manufacturers and distributors must consider when they plan to manufacture medical devices including medical device kits trays or packs for distribution in the United States
Templates are pre-designed files or files that can be used for different purposes. They can save effort and time by supplying a ready-made format and layout for producing various type of content. Templates can be used for personal or expert projects, such as resumes, invitations, leaflets, newsletters, reports, discussions, and more.
Medical Device Quality Systems Manual A Small Entity Compliance Guide

ISO 13485 2003 Quality Management System For Medical Devices QMS For

Medical Device Quality Assurance Certificate

Quality Manual Template Example Iso 9000 Quality Management System

QMS Documentation For Medical Devices ISO 13485 Certification IZiel
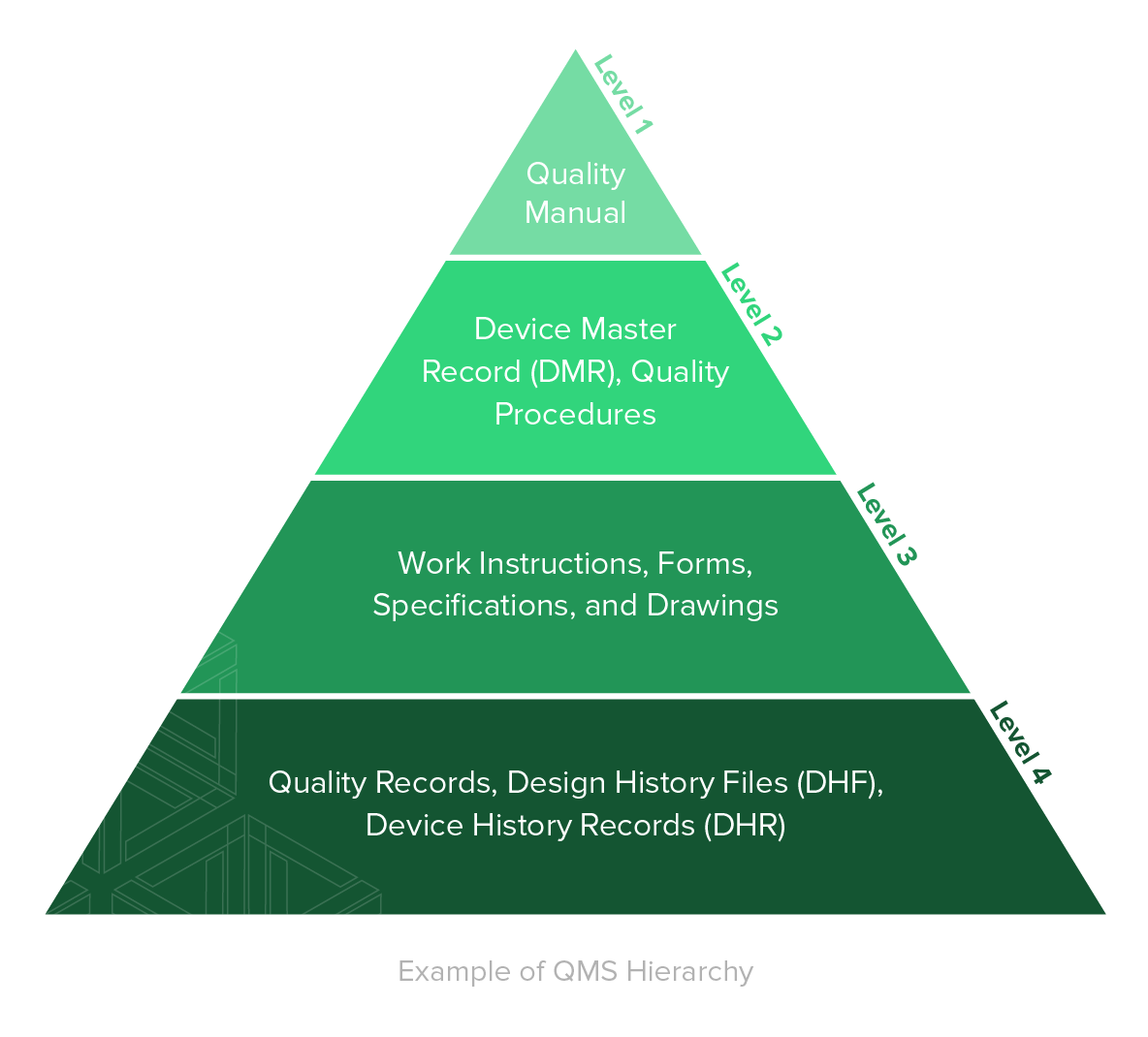
Ultimate Guide To ISO 13485 Quality Management System QMS For Medical

Medical Device Quality Management System Arrotek Arrotek

https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/inspection-guides/quality-systems
The Federal Food Drug and Cosmetic Act The Safe Medical Devices Act SMDA of 1990 and the Medical Device Amendments of 1992 Medical Device Quality Systems Manual A Small Entity Compliance Guide

https://ntrl.ntis.gov/NTRL/dashboard/searchResults/titleDetail/PB97148001.xhtml
This manual covers requirements of the Quality System regulation that manufacturers of medical devices must consider when they design devices or when they manufacture contract manufacture remanufacture process repack or relabel finished medical devices intended to be commercially distributed
https://www.fda.gov/media/76038/download
Guide to Inspections of Quality Systems 4 may be used to assess a medical device manufacturer s compliance with the Quality r Medical Device Quality Systems Manual A Small Entity

https://archive.org/details/fdasmedicaldevic00arli
Reprint of Medical device quality systems manual a small entity compliance guide 1st ed Rockville Md U S Dept of Health and Human Services Public Health Service Food and Drug Administration Center for Devices and Radiological Health 1996 Medical devices HHS publication no FDA 97 4179 Access restricted item true Addeddate

https://searchworks.stanford.edu/view/3464126
1st ed Imprint Rockville Md U S Dept of Health and Human Services Public Health Service Food and Drug Administration Center for Devices and Radiological Health 1996 Physical description vi 434 p ill 28 cm Series Medical devices HHS publication FDA 97 4179 At the library Green Library Today s hours 8a 12a
This guidance is intended primarily to provide information to the medical device industry including small businesses concerning FDA s September 24 2013 final rule establishing a unique 1st ed by Andrew Lowery 0 Ratings 0 Want to read 0 Currently reading 0 Have read This edition doesn t have a description yet Can you add one Publish Date 1996 Publisher U S Dept of Health and Human Services Public Health Service Food and Drug Administration Center for Devices and Radiological Health Language English Subjects
Medical Devices Medical Device Quality Systems Manual A Small Entity Compliance Guide First Edition supersedes the Medical Device Good Manufacturing Practices GMP manual this page provides information and access to this CDRH manual as individual chapters as shown below Copyright Attribution Non Commercial BY NC Available Formats