Chapter 3 Study Guide Matter Properties And Changes Standardized Test Practice Chapter Test Problem of the Week WebQuest Online Activity Links Section 1 Properties of Matter Section 2 Changes in Matter Section 3 Mixtures of Matter Section 4 Elements and Compounds
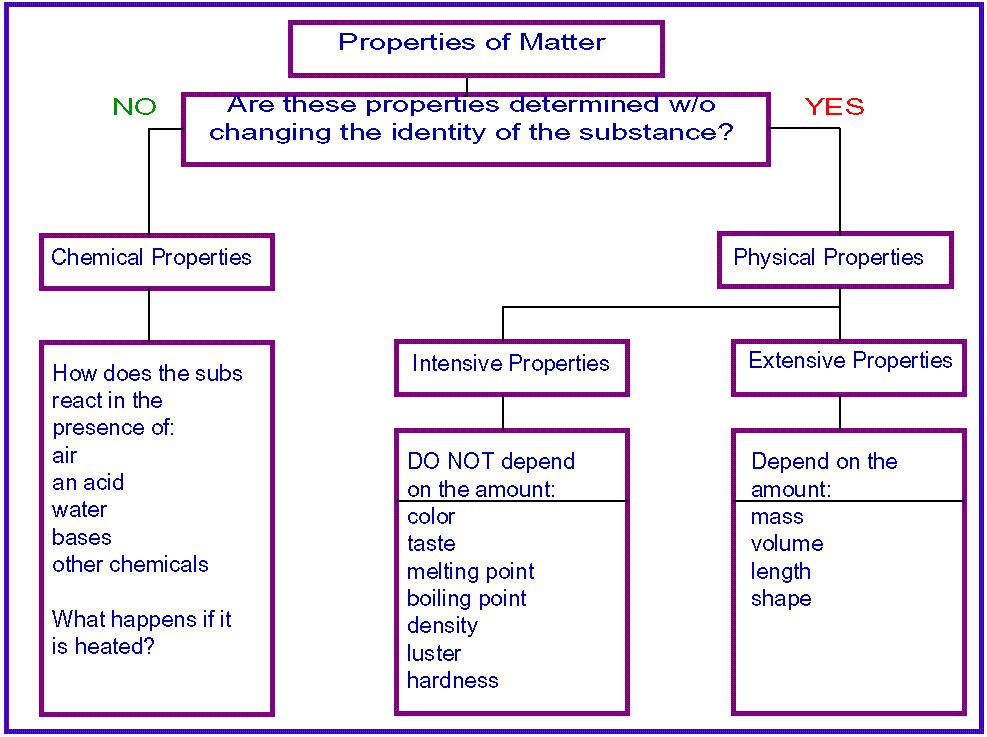
Chapter 3 continued Chemistry Matter and Change Solving Problems A Chemistry Handbook 26 3 3 Mixtures of Matter A mixture is a combination of two or more pure substances in which each substance retains its individual properties Concrete most rocks most metal objects all food and the air you breathe are mixtures that are Name CHAPTER Matter Date STUDY GUIDE Class Properties and Changes Section 3 1 Properties of Matter In your textbook read about physical properties and chemical properties of matter Use each of the terms below just once to complete the passage chemical density Matter is anything with 1 mass properties physical substance and volume
Chapter 3 Study Guide Matter Properties And Changes
 Chapter 3 Study Guide Matter Properties And Changes
Chapter 3 Study Guide Matter Properties And Changes
https://1.bp.blogspot.com/-EF2sXt8Mmaw/XXK4LPHAWvI/AAAAAAAArdA/bZ-mAExDFH0o58hlsjzL0cMnGsPUxqB1wCLcBGAs/s1600/Ch%2B2%2BNotes_85.jpeg
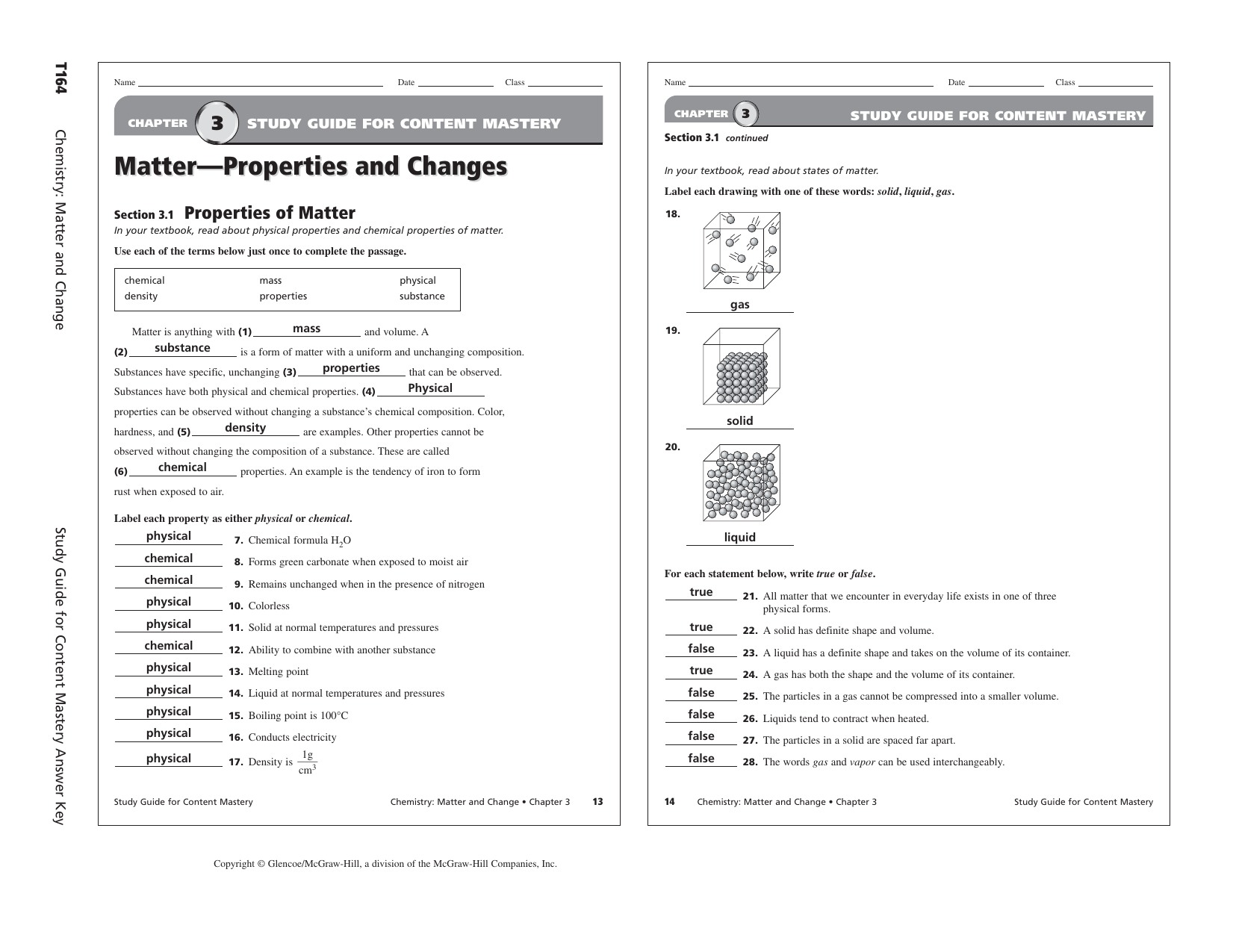
CHAPTER 3 STUDY GUIDE FOR CONTENT MASTERY Chemistry Matter and Change Section 3 1 continued Matter Properties and Changes In your textbook read about states of matter Label each drawing with one of these words solid liquid gas Section 3 1 Properties of Matter 18
Pre-crafted templates use a time-saving option for developing a diverse variety of documents and files. These pre-designed formats and layouts can be made use of for different personal and expert jobs, including resumes, invites, leaflets, newsletters, reports, discussions, and more, simplifying the material creation process.
Chapter 3 Study Guide Matter Properties And Changes

Chapter 3 Matter Properties And Changes
/chemical-properties-of-matter-608337-v33-5b6334d346e0fb0082054666.png)
Chemical Properties Of Matter

Properties Of Matter Anchor Chart For School Pinterest Anchor

Chapter 3 Matter Properties And Changes

Study Notes For Physical Properties Of Matter
Properties Of Matter Science 6 At FMS

https://www.gpschools.org/cms/lib/MI01000971/Centricity/Domain/478/glencoe%20matter%20properties%20p68%2069%2070%201314.pdf
68Chemistry Matter and Change Chapter 3 Study Guide Matter Properties and Changes Section 3 1Properties of Matter In your textbook read about physical properties and chemical properties of matter Use each of the terms below just once to complete the passage Matter is anything with 1 and volume A
https://glencoe.mheducation.com/sites/007874637x/student_view0/chapter3/
Careers in Chemistry Concepts in Motion Interactive Tutor Personal Tutor Vocabulary eFlashcards Section 1 Properties of Matter Section 2 Changes in Matter Section 3 Mixtures of Matter Section 4 Elements and Compounds

https://quizlet.com/110513409/chapter-3-matter-properties-and-changes-study-guide-flash-cards/
Law os Conservation of Energy states that energy can be converted from one form to another but it can not be created or destroyed in physical or chemical changes Mass is a form of energy states by Albert Einstein in 1905 E mc 2 E energy measured in joules M mass measured in klograms C speed of light 3 0x10 8 m s
https://glencoe.mheducation.com/sites/007874637x/student_view0/chapter3/standardized_test_practice.html
C compounds elements D heterogeneous mixtures compounds 15 Sucrose is composed of carbon hydrogen and oxygen Which of the following data is not needed in order to determine the percent by mass of the three elements

https://glencoe.mheducation.com/sites/0078617650/student_view0/chapter3/chapter_review_quizzes-eng_.html
Physical Science The Nature of Matter Book K Chapter 3 Properties and Changes of Matter Properties and Changes of Matter Your Results The correct answer for each question is indicated by a 1 When you observe a physical property the substance Need a Hint A changes B melts C does not change
Name Chapter 3 Matter 1 Chapter 3 Matter Properties and Changes State of matter also known as a Elements and compounds can move from one phase to another phase when special physical forces are present One example of those forces is temperature When temperature changes the phase can also change Solid 56 Chapter 3 Matter Properties and Changes Figure 3 1 Miners relied on the physical property of density to distin guish gold 19 g cm3 from the worthless minerals in their sluice pans The density of pyrite a worthless mineral often mis taken for gold is 5 g cm3 Physical Properties of Common Substances State Melting Boiling Density
Matter is anything with 1 2 and volume A is a form of matter with a uniform and unchanging composition Substances have specific unchanging 3 that can be observed Substances have both physical and chemical properties 4 properties can be observed without changing a substance s chemical composition Color hardness and 5