Chapter 5 Study Guide Chemistry Chapter Test Practice Careers in Chemistry Concepts in Motion Interactive Tutor Personal Tutor Vocabulary eFlashcards Section 1 Light and Quantized Energy Section 2 Quantum Theory and the Atom Section 3 Electron Configurations
Created by sarina keomek Terms in this set 55 Energy The ability to do work or cause change Amplitude Height of a wave Frequency the number of complete wavelengths that pass a point in a given time Hertz Hz Unit of measurement for frequency light one type of electromagnetic radiation Examples X rays radio waves and microwaves wave CH5 Problem 1P Step by step solution Step 1 of 2 5 gaseous elements are 1 H 2 Hydrogen 2 O 2 Oxygen 3 N 2 Nitrogen 4 He Helium 5 Ne Neon Step 2 of 2 5 gaseous compounds are 1 CO 2 Carbon dioxide 2 CO Carbon monoxide 3 N 2 O Nitrous Oxide
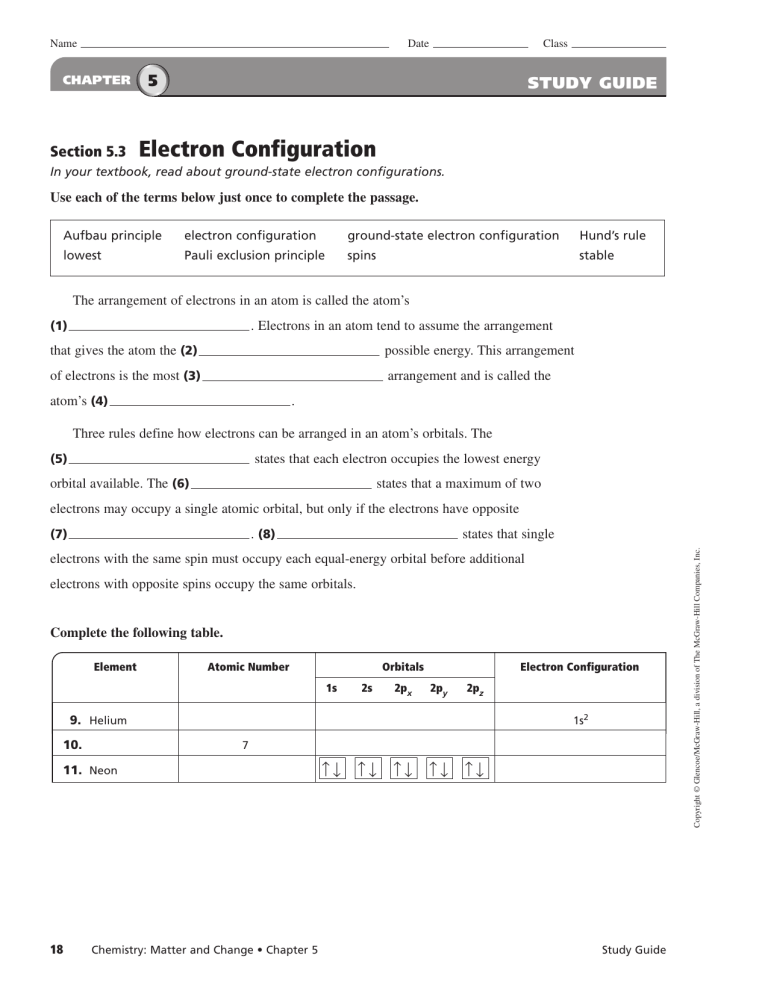
Chapter 5 Study Guide Chemistry
 Chapter 5 Study Guide Chemistry
Chapter 5 Study Guide Chemistry
https://s3.studylib.net/store/data/008715180_1-bc33de00ea18a45e91dce19394afd2ed.png
Study Guide for An Introduction to Chemistry Exercise 5 2 Classifying Compounds Classify each of the following substances as either a molecular compound or an ionic compound Obj 9 a formaldehyde CH 2 O used in embalming fluids all nonmetal atoms molecular b magnesium chloride MgCl 2 used in fireproofing wood and in paper
Pre-crafted templates use a time-saving solution for producing a varied range of documents and files. These pre-designed formats and layouts can be used for different individual and professional jobs, consisting of resumes, invites, flyers, newsletters, reports, presentations, and more, simplifying the content development process.
Chapter 5 Study Guide Chemistry

Physical Science Ms Clayton Chapter 5 Study Guide

Chapter 5 Study Guide Chapter 5 Tissues Study Guide Define TISSUE

Chapter 5 Study Guide

Chapter 5 Study Guides

Modern Chemistry Chapter 1 Test

Chapter 3 Study Guide

https://quizlet.com/538918336/chemistry-chapter-5-study-guide-flash-cards/
Chemistry Chapter 5 Study Guide 5 0 1 review Write the electron configuration for an arsenic atom Calculate the total number of electrons in each energy level and state which energy levels are not full Click the card to flip 1s 2 2s 2 2p 6 3s 2 3p 6 3d 10 4s 2 4p 3 n 1 2 electrons n 2 8 electrons n 3 18 electrons and n 4 5 electrons

http://mchsapchemistry.com/uploads/7/7/1/5/7715124/ch_5_study_guide.pdf
1 Explain the roles of Mendeleev and Moseley in the development of the periodic table 2 Describe the modern periodic table 3 Explain how the periodic law can be used to predict the physical and chemical properties of elements 4 Describe how the elements belonging to a group of the periodic table are interrelated in terms of atomic elements
https://quizlet.com/65676226/chemistry-chapter-5-study-guide-flash-cards/
Created by Kendra Gardner Terms in this set 28 Periodic Repeating patterns Periodic Law The physical and chemical properties of the elements that are periodic functions of their atomic numbers Periodic Table Arrangement of the elements by their properties Modern Periodic Table Since Mendeleev

https://quizlet.com/15287431/chemistry-chapter-5-study-guide-flash-cards/
Chemistry Chapter 5 Study Guide Term 1 29 Mendeleev Click the card to flip Definition 1 29 Noticed that when elements were arranged in order of increasing atomic mass certain similarities in their chemical properties appeared at regular intervals Click the card to flip Flashcards Learn Test Match Created by theduker6

https://quizlet.com/158362992/chapter-5-electrons-in-atoms-study-guide-flash-cards/
Chapter 5 Electrons in Atoms Study Guide 5 0 2 reviews Get a hint Electromagnetic radiation is a kind of that behaves like a n as it travels through space Click the card to flip energy wave Click the card to flip 1 43 Flashcards Learn Test Match Q Chat MandyW2015 Top creator on Quizlet Students also viewed Gilded Age
Solutions Introduction to Chemical Solutions Composition of Solutions Colligative Properties of Solutions Solubility Review of Chemical Solutions Acids Bases Introduction to Acids and Bases Fundamentals of Acids and Bases pH Calculations Buffers Titrations Review of Acids and Bases Electrochemistry Introduction to Electrochemistry 50 Study Guide for An Introduction to Chemistry Section Goals and Introductions Section 5 1 Acids Goals To describe acids To make the distinction between strong and weak acids To show the changes that take place on the particle level when acids dissolve in water To show how you can recognize strong and weak acids This section introduces one way to define acids called the Arrhenius definition
Chapter 5 Terminology Thermodynamics Thermochemistry System First law of thermodynamics Internal energy Surroundings Endothermic Exothermic State function Pressure volume work Enthalpy Enthalpy of reaction Calorimetry Calorimeter Heat capacity Molar heat capacity Specific heat Bomb calorimeter Constant pressure calorimetry Hess s law Enthalpy of