Stoichiometry Chapter 11 Study Guide Stoichiometry The study of quantitative relationships between the amounts of reactants used and amounts of products formed by a chemical reaction Law of Conservation of Mass Based on the law of conservation of mass matter is neither destroyed nor created in a chemical reaction In any chemical reaction the amount of matter present at the
A stoichiometric quantity of chlorine gas is added to an aqueous solution of NaBr to produce an aqueous solution of sodium chloride and liquid bromine Write the chemical equation for this reaction Then assume an 89 yield and calculate the mass of chlorine given the following 9 36 10 24 formula units of NaCl The starting substance in a chemical reaction stoichiometry the study of quantitative relationships between the amounts of reactants used and amounts of products formed by a chemical reaction mole ratio a ratio between the numbers of moles of any two of the substances in a balanced chemical equation derive to obtain from a specified source
Stoichiometry Chapter 11 Study Guide
 Stoichiometry Chapter 11 Study Guide
Stoichiometry Chapter 11 Study Guide
https://i.ytimg.com/vi/k1RSc4anZS4/maxresdefault.jpg
CHEM 2000 Chemistry for Engineers Sinex Unit 4 Nomenclature and Reactions Chapter 11 Stoichiometry Expand collapse global location
Pre-crafted templates provide a time-saving solution for developing a varied range of documents and files. These pre-designed formats and designs can be used for numerous individual and professional jobs, consisting of resumes, invitations, leaflets, newsletters, reports, presentations, and more, simplifying the material production process.
Stoichiometry Chapter 11 Study Guide

Chemistry Class 11 Notes Perfect 24 U

Stoichiometry

Chapter 12 Stoichiometry

Stoichiometry Chemistry Lessons Chemistry Education Chemistry Notes

Stoichiometry

Intro To Stoichiometry WS

https://quizlet.com/9053686/stoichiometry-chapter-11-flash-cards/
1 33 Created by SnydoramaTeacher Share 11 1 Defining Stoichiometry 11 2 Stoichiometric Calculations 11 3 Limiting Reactants 11 4 Percent Yield Share Stoichiometry Test Chapter 11 14 terms sylviewiley Preview Fahrenheit 451 Study Guide Questions 28 terms alypast15 Preview Chemistry Chapter 11 Stoichiometry 15 terms heididunne Preview
https://www.madison-schools.com/cms/lib9/MS01001041/Centricity/Domain/3318/chapter_11_-_stoichiometry.pdf
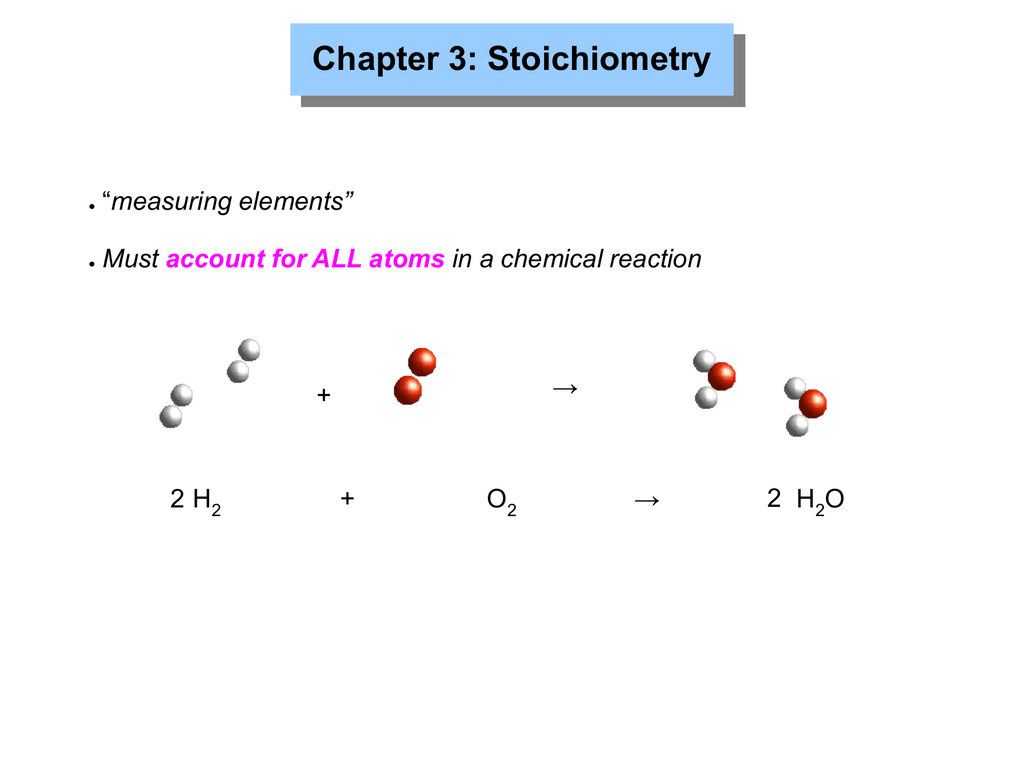
Stoichiometry is the tool for answering these questions Stoichiometry The study of quantitative relationships between the amounts of reactants used and amounts of products formed by a chemi cal reaction is called stoichiometry Stoichiometry is based on the law of conservation of mass Recall from Chapter 3 that the law states that

https://www.livingston.org/cms/lib4/NJ01000562/Centricity/Domain/911/Ch%2012%20Stoichiometry%20Notes%20Pkt%2012-13.doc
CHAPTER 11 STOICHIOMETRY 4 Balance the equation K3PO4 Al NO3 3 KNO3 AlPO4 two moles of potassium phosphate react with two moles of aluminum nitrate VOLUME to VOLUME problems Given the volume of one substance gas at STP and asked to find the volume of another substance gas in a RXN

https://chem.libretexts.org/Courses/Prince_Georges_Community_College/CHEM_2000%3A_Chemistry_for_Engineers_(Sinex)/Unit_4%3A_Nomenclature_and_Reactions/Chapter_11%3A_Stoichiometry/Chapter_11.1%3A__Again_the_Mole
Similarly the mass of 1 mol of helium atomic mass 4 002602 amu is 4 002602 g which is about one third that of 1 mol of carbon 12 Using the concept of the mole we can now restate Dalton s theory 1 mol of a compound is formed by combining elements in amounts whose mole ratios are small whole numbers
https://www.sparknotes.com/chemistry/stoichiometry/stoichiometriccalculations/
From a general summary to chapter summaries to explanations of famous quotes the SparkNotes Stoichiometric Calculations Study Guide has everything you need to ace quizzes tests and essays Search all of Renew your subscription to regain access to all of our exclusive ad free study tools Renew your subscription Please wait while we
372 Chapter 11 Stoichiometry Self Check Quiz glencoe Section Summary Balanced chemical equations can be interpreted in terms of moles mass and representative particles atoms molecules formula units The law of conservation of mass applies to all chemical reactions Mole ratios are derived from the coef ficients of a balanced chemical Stoichiometry is the study of quantitative relationships between amounts of reactants used and amounts of products formed by a chemical reaction Mole RATIOS are used as conversion factors to convert the known number of moles of one substance to the unknown numbers of moles in the same reaction Save Save chapter 11 stochiometry For Later
Chemistry Chapter 11 Stoichiometry Term 1 15 Stoichiometry Click the card to flip Definition 1 15 the study of the Quantitative or measurable relationships that exist in chemical formulas and chemical reactions Click the card to flip Flashcards Learn Test Match Created by heididunne Terms in this set 15 Stoichiometry