Standard Process Clinical Reference Guide Keeping the coverage practical the author focuses on the most critical information that impacts clinical trial conduct providing a full end to end overview for clinical data managers Features Provides an introduction and background information for the spectrum of clinical data management tasks
The original Veterinary Clinical Reference Guide was developed as a tool for veterinarians It describes clinical applications for Standard Process SP whole food nutritional supplements originally printed in 1999 WHO guidelines The development of global guidelines ensuring the appropriate use of evidence represents one of the core functions of WHO A WHO guideline is defined broadly as any information product developed by WHO that contains recommendations for clinical practice or public health policy
Standard Process Clinical Reference Guide

https://imgv2-1-f.scribdassets.com/img/document/364858803/original/65043f3bff/1582682955?v=1
This manual updated in April 2020 describes our process to develop high standard clinical guidelines and includes information on grading evidence consensus methods dissemination and implementation
Pre-crafted templates provide a time-saving solution for developing a diverse series of files and files. These pre-designed formats and layouts can be used for different personal and expert jobs, consisting of resumes, invites, flyers, newsletters, reports, presentations, and more, simplifying the content development procedure.
Standard Process Clinical Reference Guide
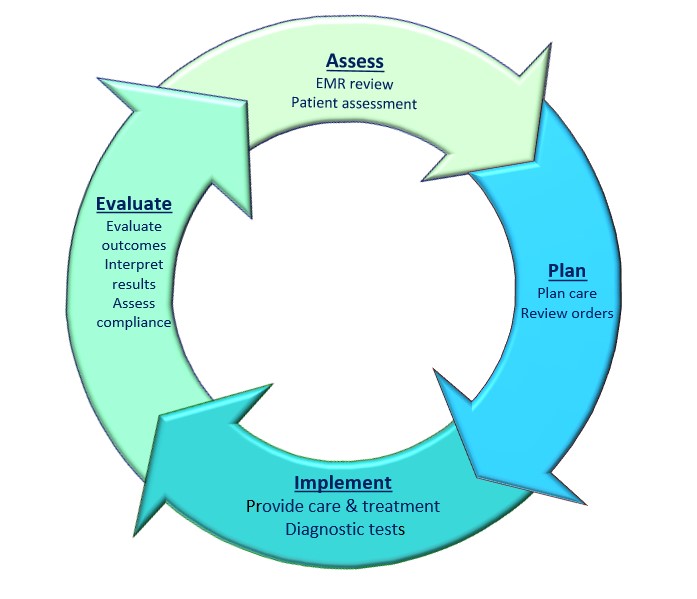
Nursing Guidelines Nursing Documentation Principles

Printable Medical Coding Cheat Sheet
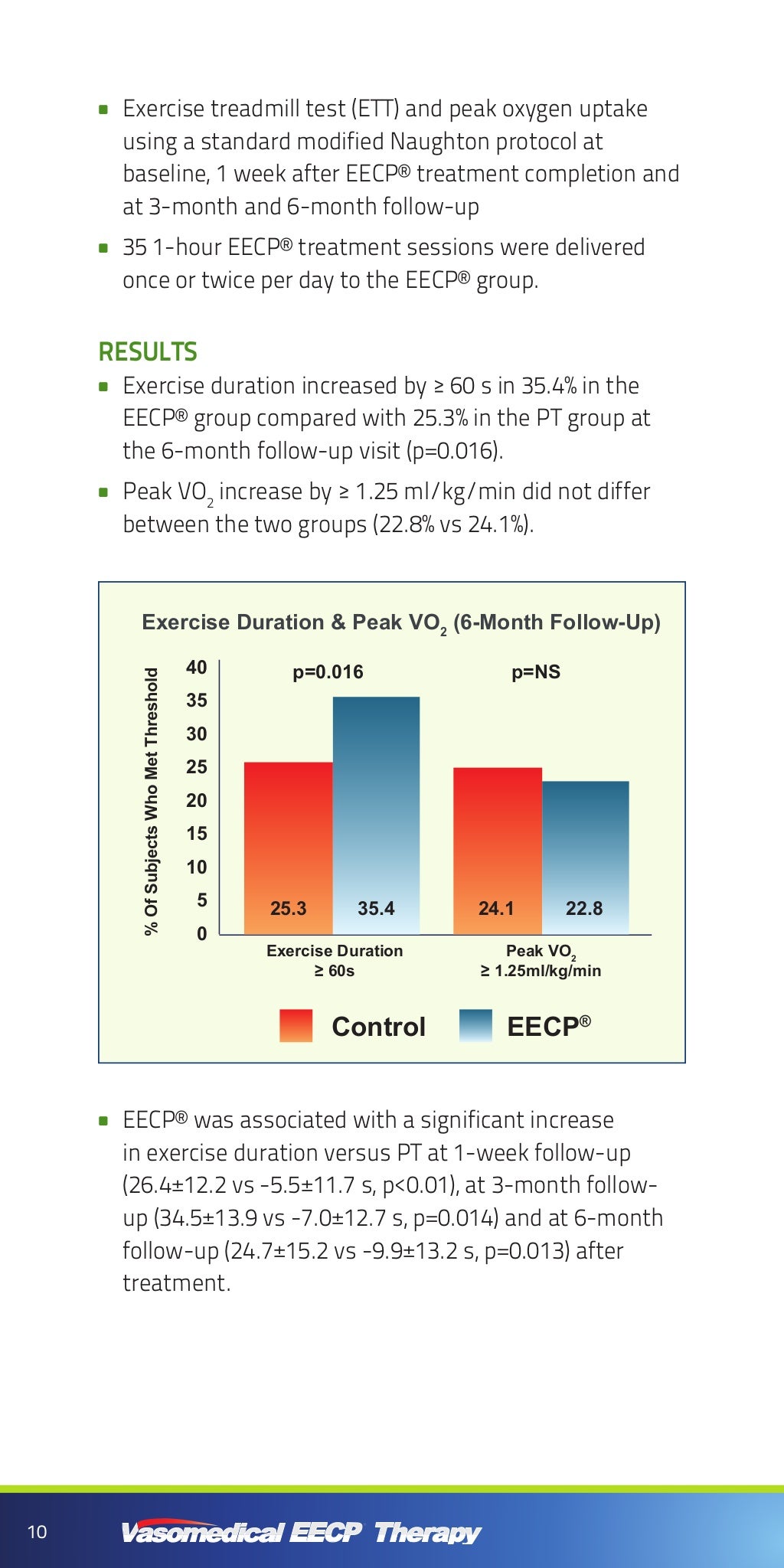
EECP Clinical Reference Guide

Heart Failure Medication Chart

Otolaryngology Head And Neck Surgery Rapid Clinical And Board Review

The 7 Steps Of The Standards Development Process HSO Health Standards

https://ifnh.org/product/pre-sales-clinical-reference-guide-crg-fall-2022
The Clinical Reference Guide is one of the textbooks for our Certified Clinician of Whole Food Nutrition CCWFN It contains the test you need to figure out the root cause of your clients concerns It includes all the tests one learns in the program Barnes Thyroid Test Iodine Patch Test Zinc Taste Test Ragland s Test etc

https://wiki.drf.com/content/detail/Download_PDFS...
Operations this user friendly reference guides the reader through each step of the clinical trial process from site selection to site set up subject recruitment study visits and to

https://wiki.drf.com/content/Resources/Documents...
Reference guide to understanding and applying the rules for properly conducting clinical trials to meet the international quality standard Good Clinical Practice provided by the

https://unshealthstore.com/books/standard-process...
Your complete guide to everything you want to know about the Standard Process and Medi Herb line of supplements including a complete list of ingredients of every single product Login or Register to write a review
https://my.standardprocess.com/flipbooks/sp-guide/index.html
August 2019 SP Product Guide Thumbnails Auto Flip Share August 2019 SP Product Guide
The handbook is based on major international guidelines including GCP guidelines issued subsequent to 1995 such as the International Conference on Harmonization ICH Good Clinical Practice Consolidated Guideline and is organized as a reference and educational tool to facilitate understanding and imple mentation of GCP by describing Operations this user friendly reference guides the reader through each step of the clinical trial process from site selection to site set up subject recruitment study visits and to
Standard Process MediHerb supplement quick reference guide as a training tool for Practitioners and Clinical Assistants to learn about Standard Process supplements