How Does The Ideal Gas Law Work The Ideal Gas Law is a simple equation demonstrating the relationship between temperature pressure and volume for gases These specific relationships stem from Charles s Law Boyle s Law and Gay Lussac s Law Charles s Law identifies the direct proportionality between volume and temperature at constant pressure Boyle s Law identifies the inverse proportionality of pressure and
The ideal gas law also called the general gas equation is the equation of state of a hypothetical ideal gas It is a good approximation of the behavior of many gases under many conditions although it has several limitations The ideal gas law can be derived from basic principles but was originally deduced from experimental measurements of Charles law that volume occupied by a gas is proportional to temperature at a fixed pressure and from Boyle s law that for a fixed temperature the product PV PV is a constant In the ideal gas model the volume occupied by its atoms and molecules is a negligible
How Does The Ideal Gas Law Work
 How Does The Ideal Gas Law Work
How Does The Ideal Gas Law Work
https://image3.slideserve.com/6794964/ideal-gas-equation2-l.jpg
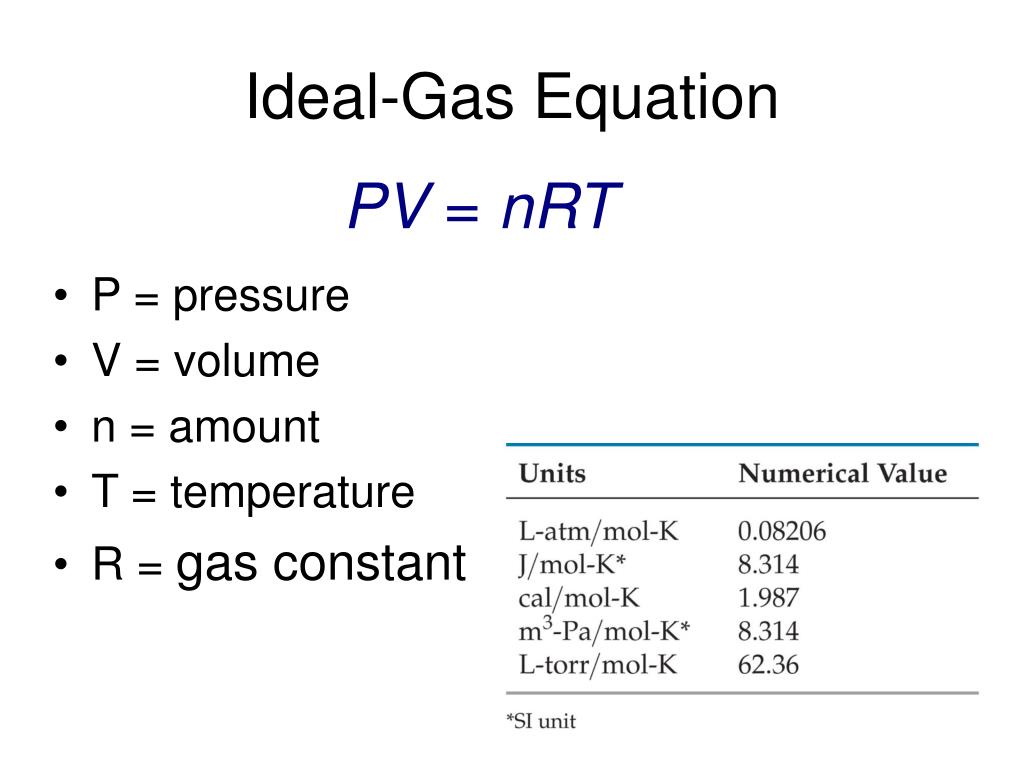
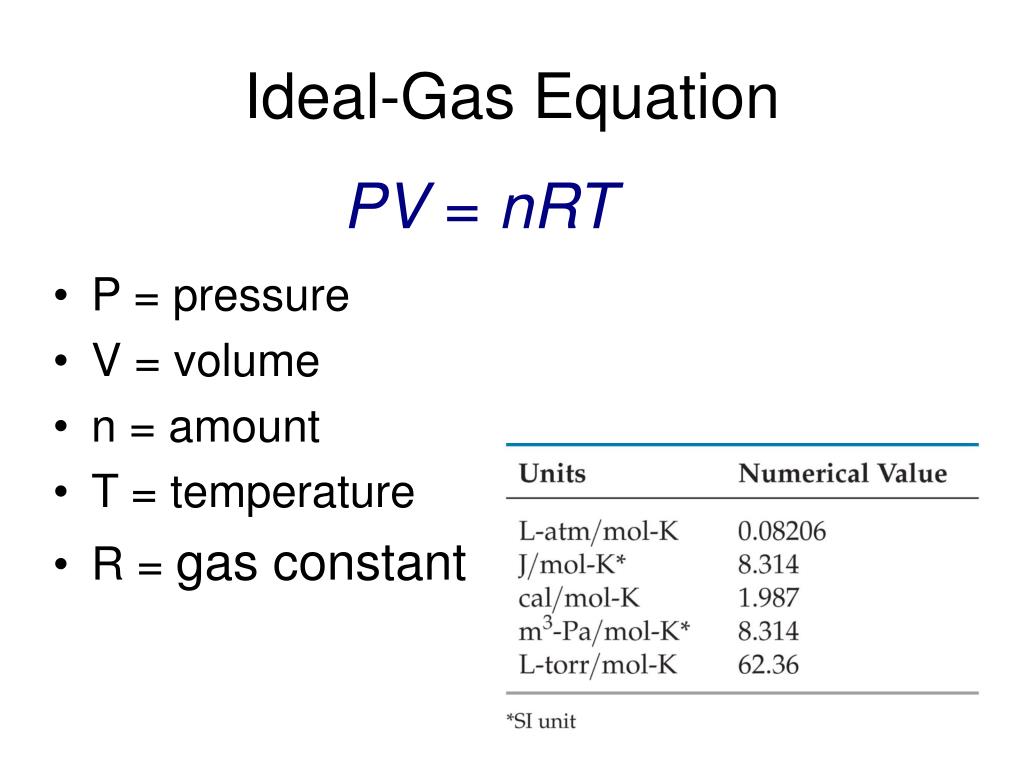
The ideal gas law provides the basis for understanding heat engines how airbags work and even tire pressure The principle equation for the ideal gas law is where is pressure is volume is the number of moles of gas is temperature measured in kelvin is the ideal gas constant The value of R depends on the units used It can also be written as where N is the number of molecules and is
Pre-crafted templates use a time-saving option for producing a varied series of files and files. These pre-designed formats and designs can be utilized for numerous individual and professional projects, consisting of resumes, invites, leaflets, newsletters, reports, presentations, and more, simplifying the content production process.
How Does The Ideal Gas Law Work

Ideal Gas Law Statement Characteristics Formula Problems

Ideal Gas Law Worksheet With Answers
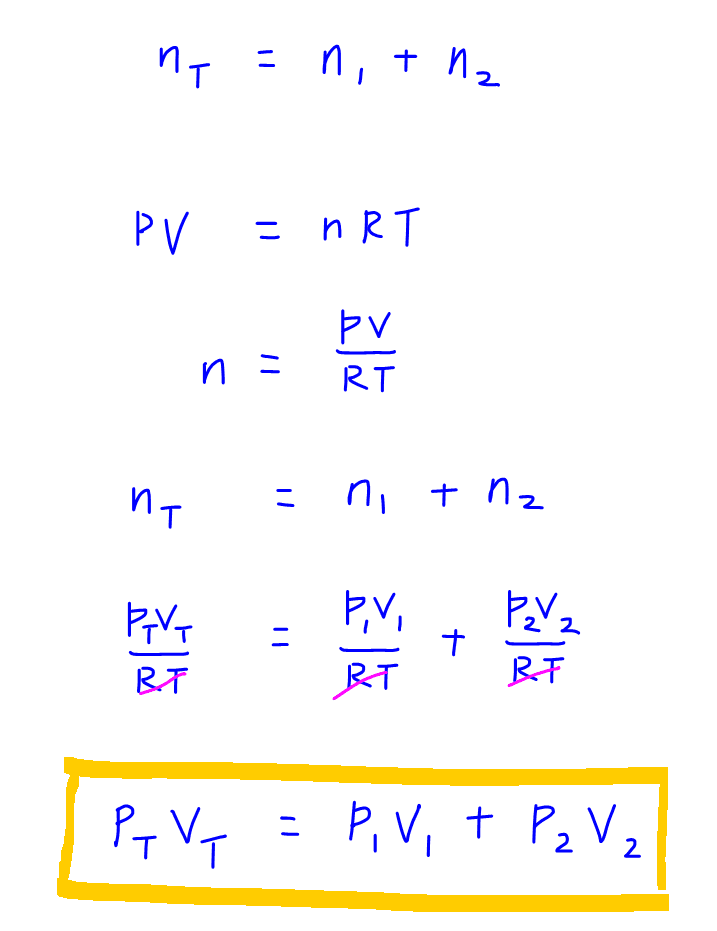
Ideal Gas Law And Applications
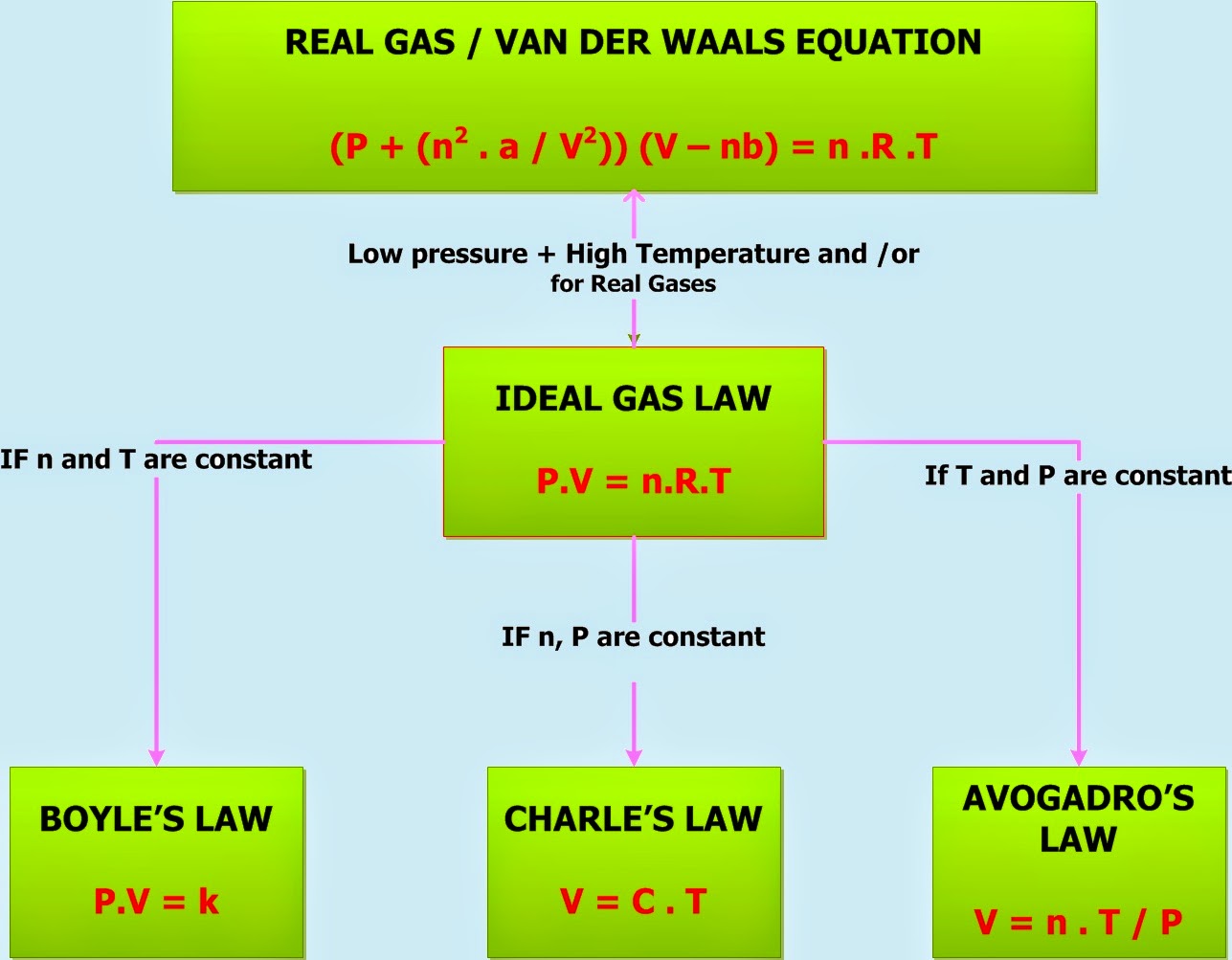
Gas Laws Ideal Gas Law Chemistry Net
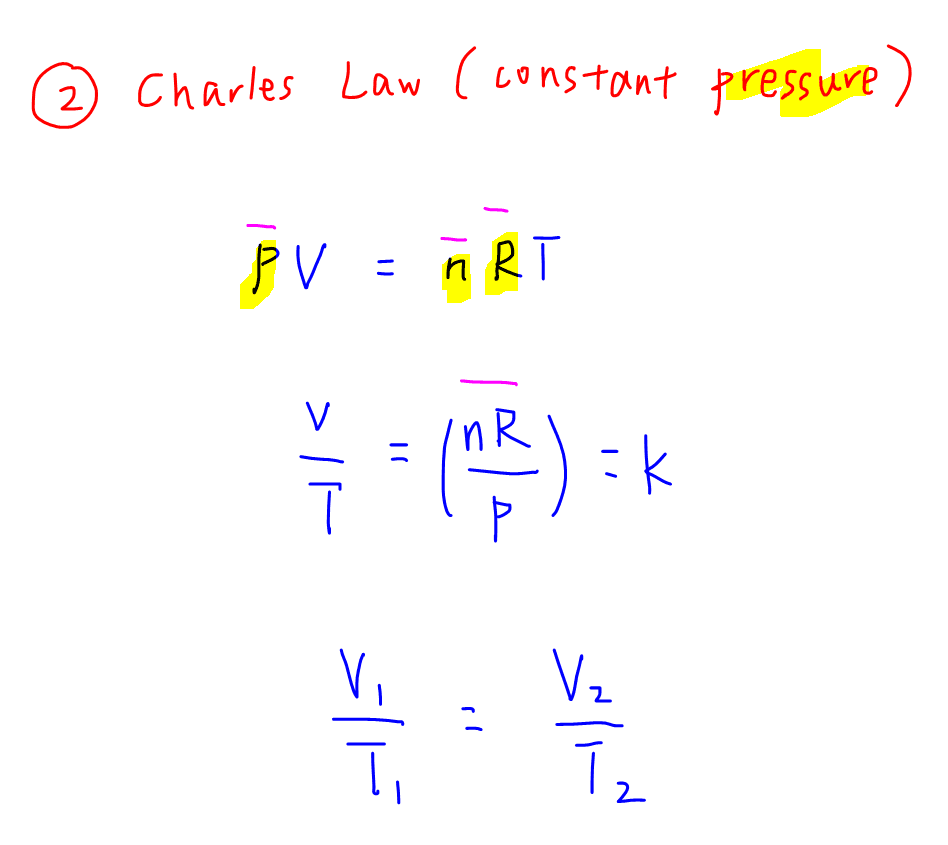
Ideal Gas Law And Applications

Teacher s Pet In Spanish The Ideal Gas Law Music History
https://www.thoughtco.com/ideal-gas-law-607531
The Ideal Gas Law may be expressed as PV NkT where P absolute pressure in atmospheres V volume usually in liters n number of particles of gas k Boltzmann s constant 1 38 10 23 J K 1 T temperature in Kelvin The Ideal Gas Law may be expressed in SI units where pressure is in pascals volume is in cubic meters N

https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Physical_Properties_of_Matter/States_of_Matter/Properties_of_Gases/Gas_Laws/The_Ideal_Gas_Law
The Ideal Gas Equation Before we look at the Ideal Gas Equation let us state the four gas variables and one constant for a better understanding The four gas variables are pressure P volume V number of mole of gas n and temperature T Lastly the constant in the equation shown below is R known as the the gas constant which will be discussed in depth further later

https://chem.libretexts.org/Courses/can/intro/11:_Gases/11.09:_The_Ideal_Gas_Law:_Pressure_Volume_Temperature_and_Moles
The Ideal Gas Law is a single equation which relates the pressure volume temperature and number of moles of an ideal gas If we substitute in the variable R for the constant the equation becomes P V T n R The Ideal Gas Law is conveniently rearranged to look this way with the multiplication signs omitted P V n R T

https://www.khanacademy.org/science/ap-chemistry-beta/x2eef969c74e0d802:intermolecular-forces-and-properties/x2eef969c74e0d802:ideal-gas-law/v/ideal-gas-equation-pv-nrt
The ideal gas law PV nRT relates the macroscopic properties of ideal gases An ideal gas is a gas in which the particles a do not attract or repel one another and b take up no space have no volume No gas is truly ideal but the ideal gas law does provide a good approximation of real gas behavior under many conditions

https://www.britannica.com/science/ideal-gas-law
The ideal gas law is a generalization containing both Boyle s law and Charles s law as special cases This law can be derived from the kinetic theory of gases and relies on the assumptions that 1 the gas consists of a large number of molecules which are in random motion and obey Newton s laws of motion 2 the volume of the molecules is negligibly small compared with the volume
The ideal gas law can also be used to determine the density of gases Density recall is defined as the mass of a substance divided by its volume d m V 6 6 1 6 6 1 d m V Assume that you have exactly 1 mol of a gas If you know the identity of the gas you can determine the molar mass of the substance There are four laws known as Gas Laws which describe how gases behave The four laws are Boyle s Law Charles s Law Gay Lussac s Law and Avogadro s Law Avogadro s Law Amadeo Avogadro was an Italian physicist who stated in 1811 that the volume of any gas is proportional to the number of molecules of gas measured in Moles symbol mol
It is summarized in the statement now known as Boyle s law The volume of a given amount of gas held at constant temperature is inversely proportional to the pressure under which it is measured Example 8 2 4 8 2 4 Volume of a Gas Sample The sample of gas has a volume of 15 0 mL at a pressure of 13 0 psi