Gas Laws Study Guide The laws of gases in chemistry are equations that express the relationship among four variables These four variables define the physical condition of a gas Temperature T pressure P
Chemistry Gas Laws Study Guide 4 3 3 reviews What are some properties of gases Click the card to flip They have mass low densities they are easily compressed completely fill their container can move through each other rapidly and exert pressure Click the card to flip 1 32 Flashcards Learn Test Match Created by Terms in this set 32 Gas laws are a set of laws that describe the relationship between pressure volume temperature and moles of gas The following relationships hold true when the amount of gas is constant Boyle s Law Boyle s law describes the relationship between the pressure and volume of an ideal gas under constant temperature
Gas Laws Study Guide
 Gas Laws Study Guide
Gas Laws Study Guide
https://classworkassignmentanswers.weebly.com/uploads/7/9/5/5/79550400/img023_5_orig.jpg
Gas laws laws that relate the pressure volume and temperature of a gas Boyle s law named for Robert Boyle states that at constant temperature the pressure P of a gas varies inversely with its volume V or PV k where k is a constant Charles s law named for J A C Charles 1746 1823 states that at constant pressure the volume V of a gas is directly proportional to
Templates are pre-designed files or files that can be used for different functions. They can conserve time and effort by providing a ready-made format and layout for producing various type of content. Templates can be used for personal or professional jobs, such as resumes, invitations, leaflets, newsletters, reports, presentations, and more.
Gas Laws Study Guide

Pin On Chemistry Notes

Gas Laws Study Guide Formative Assessment Test Bank Questions With

Ideal Gas Law Gizmo Answer Key Pdf Gas Laws Gizmo 2 docx Name Date

Pin On Chemistry Task Cards
The Gas Laws

Gas Laws Worksheet And Answers Teaching Resources

https://www.thoughtco.com/gases-study-guide-607536
Updated on July 03 2019 A gas is a state of matter with no defined shape or volume Gases have their own unique behavior depending on a variety of variables such as temperature pressure and volume While each gas is different all gases act in a similar matter

https://quizlet.com/696079198/gas-laws-study-guide-flash-cards/
Study with Quizlet and memorize flashcards containing terms like Under conditions of fixed temperature and amount of gas Boyle s law requires that Which law explains the relationship between the variable of temperature and pressure Which law explains the relationship between pressure and volume and more
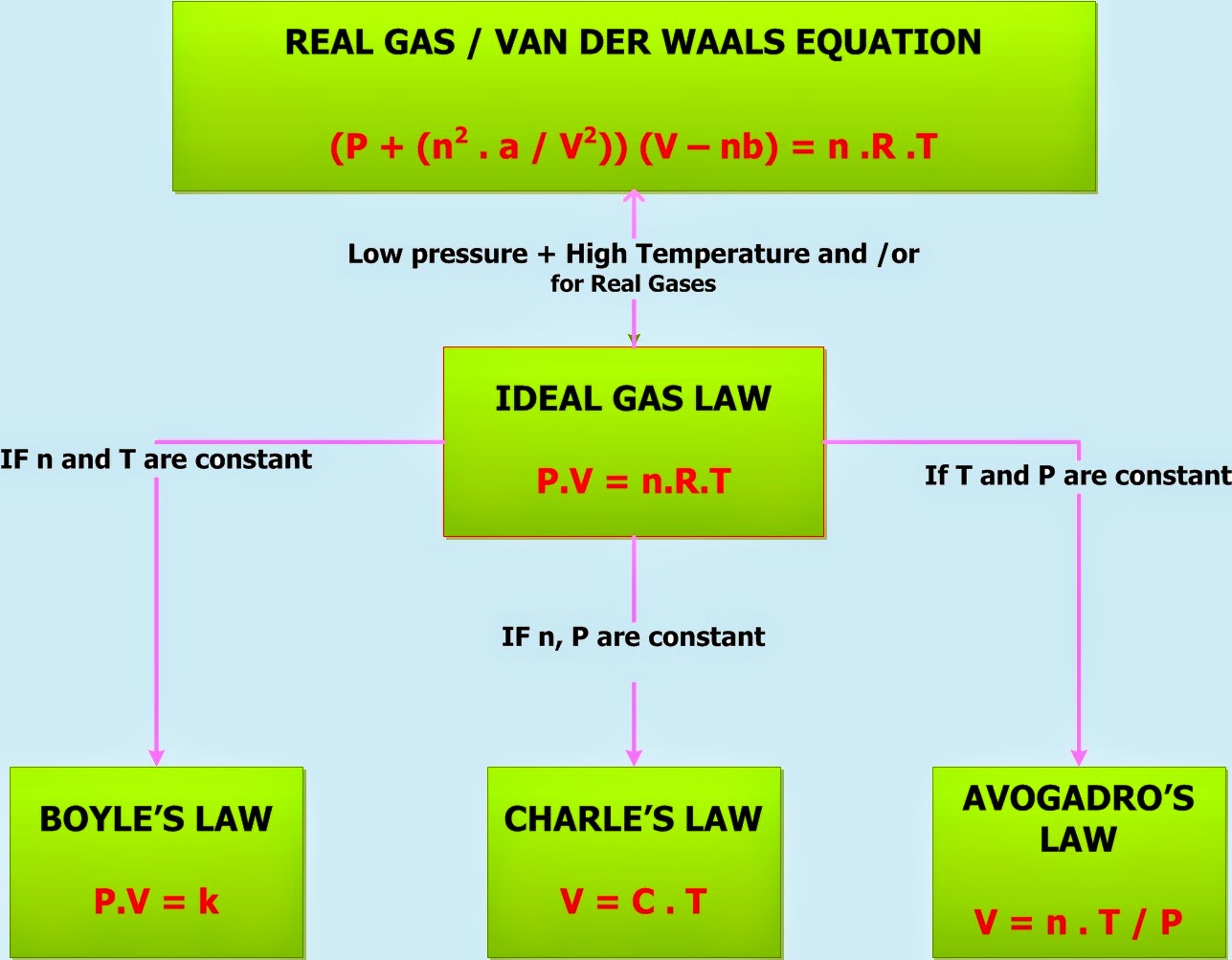
https://www.khanacademy.org/science/physical-chemistry-essentials/x98cdf762ed888601:gaseous-state/x98cdf762ed888601:gas-laws/e/gas-laws
Gas Laws Science Physical Chemistry Essentials Class 11 Gaseous state Gas Laws Gas Laws Google Classroom You might need Calculator A particular amount of ideal gas occupies 3 L at 27 o C Calculate the decrease in volume observed if the gas is cooled down to 17 o C Given The pressure remains constant L Show Calculator Stuck

https://www.studocu.com/en-us/document/studocu-university/chemistry/gas-laws-study-guide-key/59126590
Gas Laws Study Guide Test Format Multiple choice and problems Items that will be provided for the test read important notes at the bottom Gas law equations The value of R Pressure conversion factors I will provide the equations on the test You are responsible for knowing which laws the equations describe ex I will give you P 1 x V 1

https://www.sparknotes.com/chemistry/gases/ideal/
From a general summary to chapter summaries to explanations of famous quotes the SparkNotes Ideal Gases Study Guide has everything you need to ace quizzes tests and essays
The Gases Gas Laws chapter of this Thermodynamics Study Guide course is the simplest way to master gases and gas laws This chapter uses simple and fun videos that are about five The gas laws are a group of laws that govern the behaviour of gases by providing relationships between the following The volume occupied by the gas The pressure exerted by a gas on the walls of its container The absolute temperature of the gas The amount of gaseous substance or the number of moles of gas
Study Guide Gas Laws Study Guide Phases of Matter Gas Laws Also study the PowerPoints notes and all of the Daily work we did in class A What 3 units are used for temperature K C F B 1 atmosphere 760 torr 760 mm C The relationship between Temperature and Volume is proportionate or inverse D