Darzalex Faspro Billing And Coding Guide Table Of Contents Policy Applicable CPT HCPCS ICD 10 Codes Background References Policy Note Requires Precertification Precertification of daratumumab Darzalex and daratumumab and hyaluronidase fihj Darzalex Faspro are required of all Aetna participating providers and members in applicable plan designs
Are pregnant or plan to become pregnant DARZALEX FASPRO may harm your unborn baby Tell your healthcare provider right away if you become pregnant or think that you may be pregnant during treatment with DARZALEX FASPRO DARZALEX FASPRO daratumumab and hyaluronidase fihj is indicated for the treatment of adult patients with multiple myeloma In combination with bortezomib melphalan and prednisone in newly diagnosed patients who are ineligible for autologous stem cell transplant
Darzalex Faspro Billing And Coding Guide
 Darzalex Faspro Billing And Coding Guide
Darzalex Faspro Billing And Coding Guide
https://www.clinicaltrialsarena.com/wp-content/uploads/sites/22/2021/02/Image-1-DARZALEX-FASPRO-1038x720.jpg
No dose reductions of DARZALEX FASPRO are recommended Consider withholding DARZALEX FASPRO to allow recovery of blood cell counts in the event of myelosuppression see Warnings and Precautions 5 2 5 3 2 5 Preparation and Administration DARZALEX FASPRO should be administered by a healthcare provider
Pre-crafted templates provide a time-saving solution for producing a diverse series of documents and files. These pre-designed formats and designs can be used for numerous individual and professional projects, consisting of resumes, invites, leaflets, newsletters, reports, presentations, and more, streamlining the content production procedure.
Darzalex Faspro Billing And Coding Guide

First Treatment For Newly Diagnosed Light Chain Amyloidosis Approved

Understanding DARZALEX And DARZALEX FASPRO By International Myeloma

Homepage banner

FDA Approves Darzalex Faspro As Injection Therapy For Multiple Myeloma


How To Work From Home In Medical Billing And Coding Guide The
https://www.janssencarepath.com/hcp/darzalex/reimbursement/coding-billing
CONTRAINDICATIONS DARZALEX is contraindicated in patients with a history of severe hypersensitivity eg anaphylactic reactions to daratumumab or any of the components of the formulation WARNINGS AND PRECAUTIONS Infusion Related Reactions DARZALEX can cause severe and or serious infusion related reactions including anaphylactic reactions

https://www.janssencarepath.com/sites/www.janssencarepath-v1.com/files/darzalex-faspro-coding-and-billing-physician-office.pdf
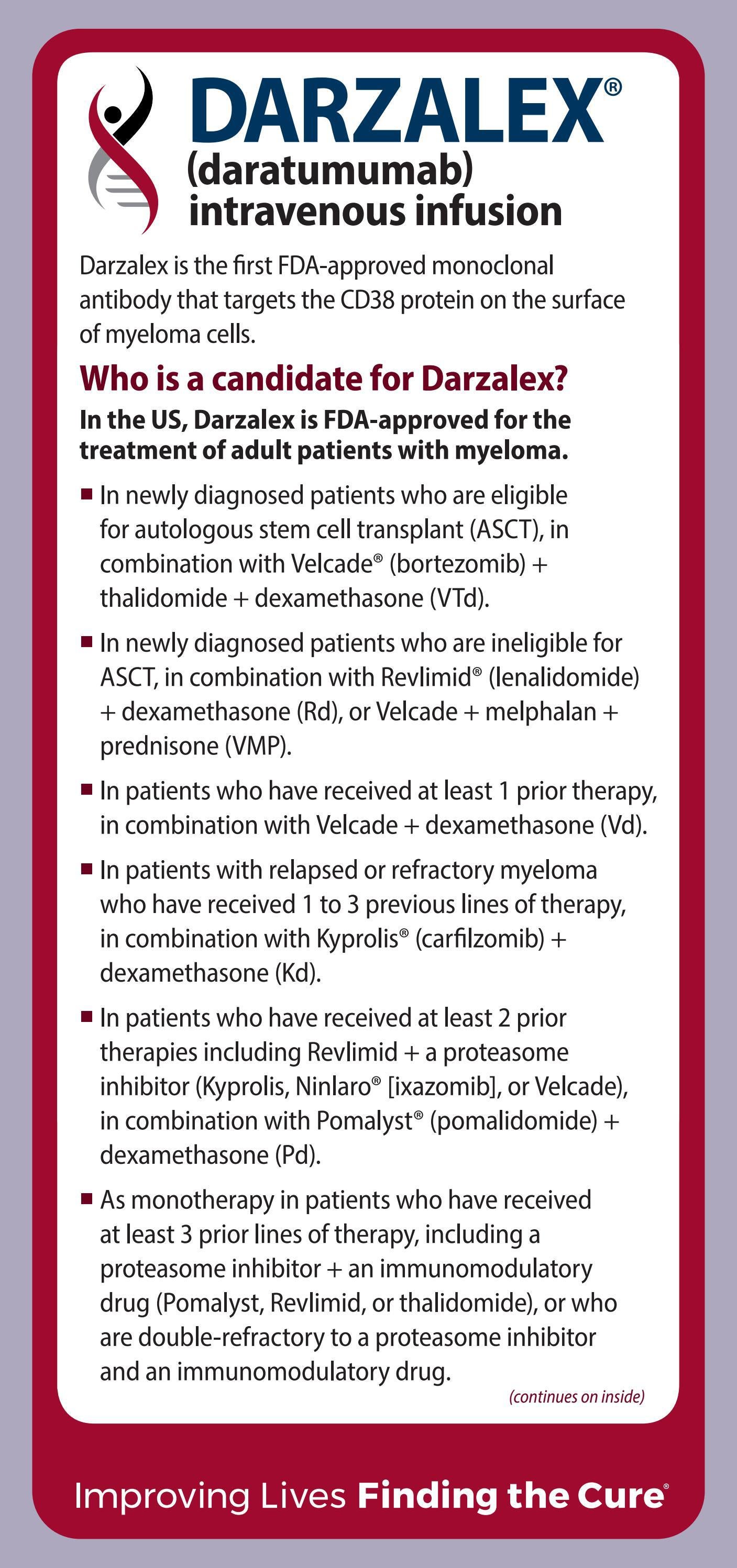
DARZALEX FASPRO daratumumab and hyaluronidase fihj is indicated for the treatment of adult patients with multiple myeloma In combination with bortezomib melphalan and prednisone in newly diagnosed patients who are ineligible for autologous stem cell transplant
https://www.janssencarepath.com/sites/www.janssencarepath-v1.com/files/darzalex-faspro-reimbursement-and-access-guide.pdf
Of the background regimen will serve as pre medication on DARZALEX FASPRO administration days Do not administer background regimen specific corticosteroids eg prednisone on DARZALEX FASPRO administration days when patients have received dexamethasone or equivalent as a pre medication Pre medications1 Coding for DARZALEX FASPRO

https://specialtydrug.magellanprovider.com/media/220898/darzalex.pdf
Used in the treatment of newly diagnosed disease in patients who are ineligible for autologous stem cell transplant ASCT in combination with ONE of the following regimens Lenalidomide and dexamethasone OR Bortezomib melphalan and prednisone OR Used as subsequent therapy in combination with dexamethasone and either lenalidomide or
https://specialtydrug.magellanprovider.com/media/262298/mrxm_darzalex_sq_01_21.pdf
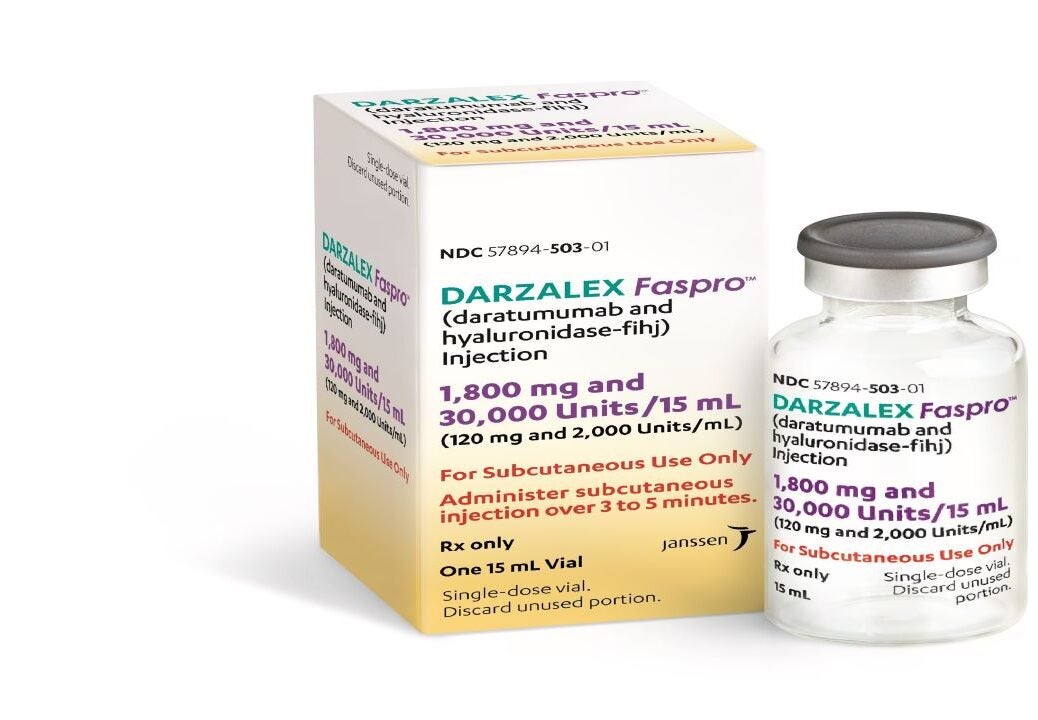
Billing Code Availability Information HCPCS Code J9999 Not otherwise classified antineoplastic drugs Discontinue use effective 1 1 21 Darzalex Faspro 1 800 mg of daratumumab and 30 000 units of hyaluronidase per 15 mL single dose vial 57894 0503 xx 2020 The NCCN Compendium is a derivative work of the NCCN Guidelines
Lancet Oncol 2021 22 11 1582 1596 View the official healthcare professional HCP website for DARZALEX DARZALEX FASPRO See full Prescribing Safety Information DARZALEX FASPRO is a CD38 targeted monoclonal antibody in a subcutaneous formulation1 DARZALEX FASPRO contains recombinant hyaluronidase which is a substance that increases permeability of subcutaneous tissue making it possible for 15 mL containing 1 800 mg of daratumumab to be administered in approximately 3 to 5 minutes 1
Patients received Darzalex Faspro 1 800 mg 30 000 units 1 800 mg daratumumab and 30 000 units hyaluronidase administered subcutaneously in combination with Kyprolis 20 70 mg m2 once weekly