Chemistry Unit 2 Study Guide Chemistry Unit 2 Study Guide Term 1 173 electromagnetic radiation Click the card to flip Definition 1 173 radiant energy that exhibits wavelike behavior as it travels through space Click the card to flip Flashcards Learn Test Match Created by jillybean48 Terms in this set 173 electromagnetic radiation
JJ Thomson developed current teaching model of the atom John Dalton said that all substances are made up of atoms John Dalton said that atoms constantly move Democritus Study with Quizlet and memorize flashcards containing terms like 3 parts of the atom location of the proton neutron and electron why are atoms neutral and more The atomic mass of an atom of carbon is 12 amu and the atomic mass of an atom of oxygen is 16 amu To produce CO 16 g of oxygen combine with 12 g of carbon To produce CO subscript 2 32 g of oxygen combine with 12 g of carbon What is the ratio of the mass of oxygen in CO subscript 2 to the mass oxygen in CO 2 1
Chemistry Unit 2 Study Guide
 Chemistry Unit 2 Study Guide
Chemistry Unit 2 Study Guide
https://www.chemistry-teaching-resources.com/images/APChem/Unit2Overview.png
Chemistry is the study of matter and the changes it undergoes Here you can browse chemistry videos articles and exercises by topic We keep the library up to date so you may find new or improved material here over time Unit 1 Atoms compounds and ions Introduction to the atom Ions and compounds Names and formulas of ionic compounds
Templates are pre-designed documents or files that can be utilized for numerous purposes. They can save time and effort by offering a ready-made format and design for creating various sort of material. Templates can be utilized for individual or professional projects, such as resumes, invites, flyers, newsletters, reports, discussions, and more.
Chemistry Unit 2 Study Guide

Chemistry Unit 1 Notes Chemistry Year 11 VCE Thinkswap
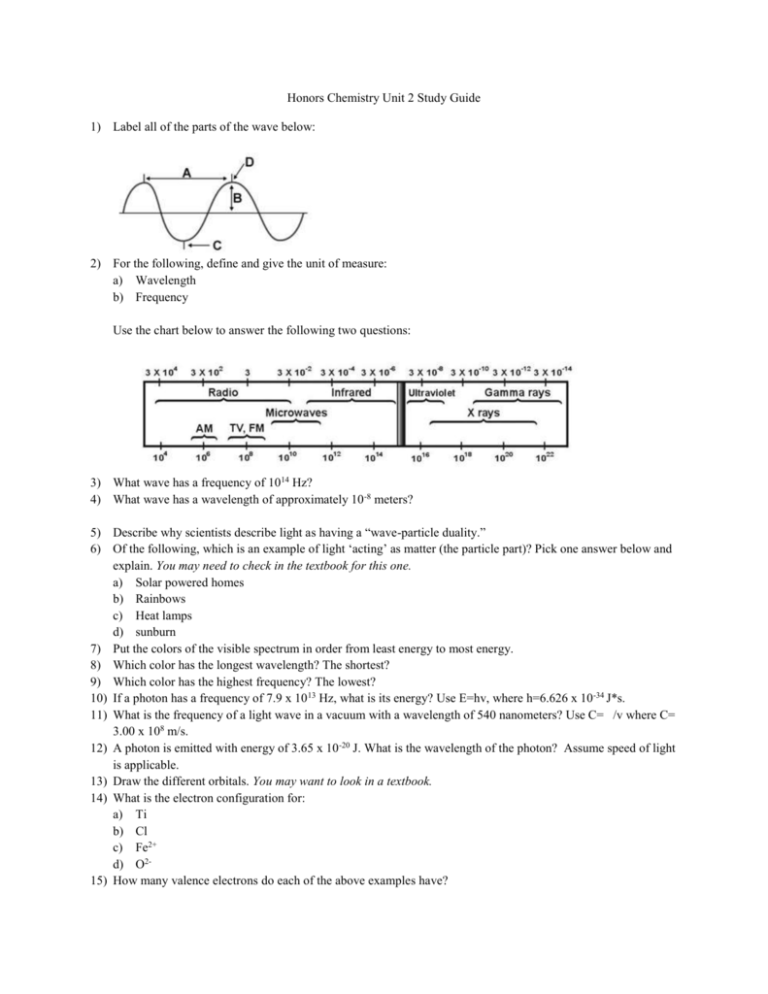
Honors Chemistry Unit 2 Study Guide Label All Of The Parts Of The

Unit 1 Study Guide Answer Key

Chemistry Unit 3 Study Guide

12th Chemistry Unit 3 Free Online Test PADAVELAI TNTET Arts

7 Best Images Of Biochemistry Review Worksheet Evolution Review

https://quizlet.com/160948941/unit-2-chemistry-flash-cards/
Electron a tiny negatively charged particle that orbits the nucleus of each atom has a mass of 9 109 382 10 28 g a negative charge of 1 602 176 10 19 coulombs and a spin of neutron a neutrally charged particle existing in the nucleus of most atoms with a mass of 1 674 927 10 24 g and a spin of

https://quizlet.com/55355309/chemistry-unit-2-study-guide-answers-flash-cards/
1 41 Flashcards Learn Test Match Q Chat Created by laniguerrero Students also viewed UNIT 2 CHEMISTRY STUDY GUIDE 178 terms nelsone17 Preview Chemistry Unit 2 Study Guide 36 terms JacobAnderson44 Preview Unit 2 Chemistry Teacher 36 terms CoachPerkins4 Preview AP World History Unit 2 Networks of Exchange Teacher 34 terms efadamson Preview

https://quizlet.com/317357383/chemistry-unit-2-study-guide-flash-cards/
Democritus first to suggest the existence of atoms John Dalton father of atomic theory discovered that oxygen and hydrogen always combine together in fixed proportions Atomic theory Dalton all matter is made up of atoms atoms can not be created or destroyed they can be in nuclear reactions

https://library.fiveable.me/ap-chem/unit-2
Unit 2 Study Guides Unit 2 Overview Molecular and Ionic Compound Structure and Properties 7 min read D written by Dalia Savy 2 1 Types of Chemical Bonds 7 min read D written by Dalia Savy 2 2 Intramolecular Force and Potential Energy 6 min read D written by Dalia Savy 2 3 Structure of Ionic Solids 7 min read D

https://www.studocu.com/en-us/document/high-school-us/chemistry/chemistry-unit-2-study-guide/8879478
Atoms of different elements are different Atoms of different elements combine in whole number ratios to form compounds Atoms of different elements are different therefore have different masses because each element has a different atomic mass 2
GENERAL CHEMISTRY EXAM 2 COMPREHENSIVE STUDY GUIDE UNIT 2 PART 1 BONDING 8 TYPES OF CHEMICAL BONDING A BONDS Forces that hold atoms together 3 types of bonds a Ionic bonds b Metallic bonds c Covalent bonds i Covalent bonds electrostatic attraction between positive nuclei and shared electrons ii Recall some steps for this process that we discussed in the last study guide Count the number of valence electrons that the molecule has in total Nitrogen has 5 and an oxygen atom has 6 Since there are three oxygen atoms we must account for each 5 6 6 6 23 But NO3 is a polyatomic ion and there is a charge attached to the molecule as
Study Guides for Every AP Chemistry Unit The AP Chemistry exam covers a ton of content When I say a ton I mean a TON The course covers everything from quantum mechanics and atomic structure to properties of states of matter to acid base titrations By the time you ve learned all of this it s more than likely that your brain is filled with