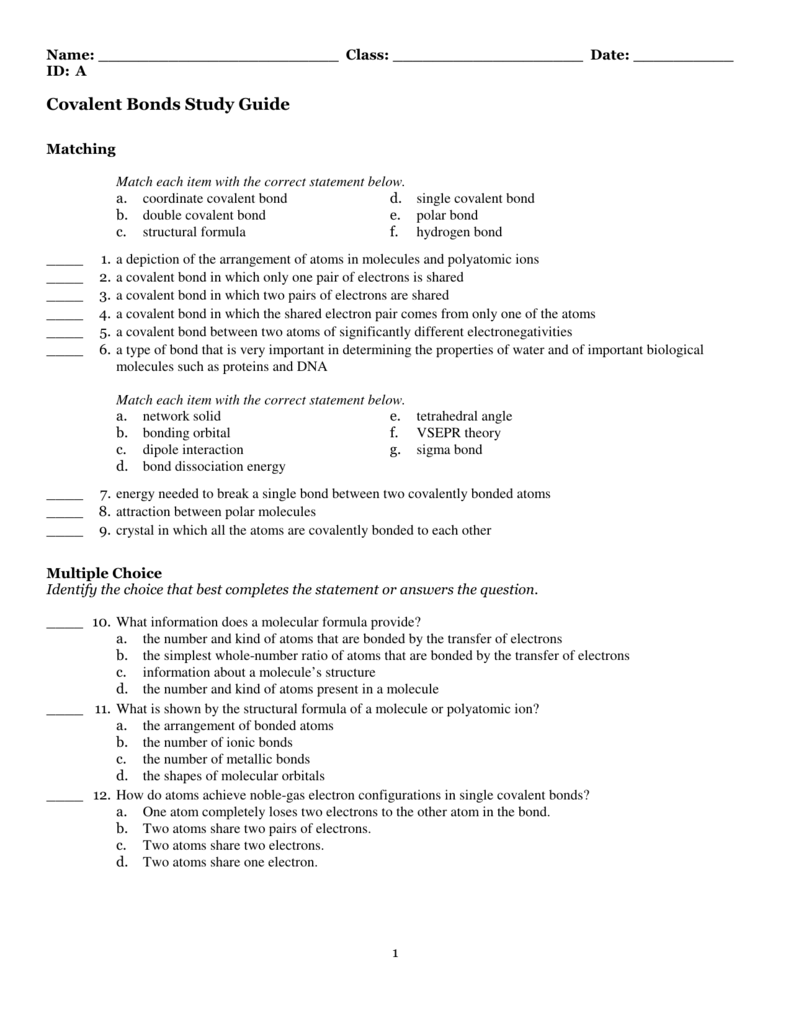
Chapter 8 Study Guide Covalent Bonding Unshared pair a pair of valence electrons that is not shared between atoms lone pair double covelant bond bond involving shared pairs of electrons triple covalent bond a bond formed by sharing three pairs of electrons coordinate covalent bond a covalent bond in which one atom contributes both bonding electrons polyatomic ion
8 3 COVALENT BONDING The strength of a covalent bond depends on the overlap between the valence orbitals of the bonded atoms Bond order is the number of electron pairs that hold two atoms together Single bonds have a bond order of one and multiple bonds with bond orders of two a double bond and three a triple bond are quite common Wikipedia 8 3 Covalent Bonding is shared under a CC BY NC SA 3 0 license and was authored remixed and or curated by LibreTexts The strength of a covalent bond depends on the overlap between the valence orbitals of the bonded atoms Bond order is the number of electron pairs that hold two atoms together
Chapter 8 Study Guide Covalent Bonding
 Chapter 8 Study Guide Covalent Bonding
Chapter 8 Study Guide Covalent Bonding
https://image.slidesharecdn.com/covalentbondingreviewsheet-110828135250-phpapp02/95/chemistry-chp-8-covalent-bonding-study-guide-1-728.jpg?cb=1314542347
Chapter 8 Covalent Bonding In this Chapter Science Fair Ideas Periodic Table Links Safety Links MSDS Links Virtual Investigations Textbook Resources Online Student Edition Multilingual Glossary Interactive Timeline Study To Go Study To Go Vocabulary eFlashcards Chapter Activities
Templates are pre-designed documents or files that can be utilized for numerous functions. They can save time and effort by offering a ready-made format and layout for producing various type of content. Templates can be utilized for individual or expert projects, such as resumes, invitations, leaflets, newsletters, reports, presentations, and more.
Chapter 8 Study Guide Covalent Bonding

Ionic Bonding And Covalent Bonding 4 GCSE Chemistry Lessons Edexcel 9

Covalent Bond Sharing Of Electrons Between Atoms Bonds Contain Energy
Covalent Bonding Worksheet Answers Soccerphysicsonline Db excel

Covalent Bonding The Science And Maths Zone

Covalent Bonding Biology Definition Role Expii
Covalent Bonding Notes2

https://quizlet.com/374096906/chapter-8-study-guide-covalent-bonding-flash-cards/
Chapter 8 Study Guide Covalent Bonding Flashcards Learn Test Match Q Chat Get a hint when sharing of electrons occurs the attachment between atoms that results is called a n Click the card to flip covalent bond Click the card to flip 1 69 1 69 Flashcards Learn Test Match Q Chat Created by jannahhazir Share Share
https://quizlet.com/250897346/chapter-8-covalent-bonding-study-guide-flash-cards/
Chapter 8 covalent bonding study guide Get a hint what are typical characteristics of an ionic compound Click the card to flip high melting and boiling point Click the card to flip 1 23 Flashcards Learn Test Match Q Chat Created by ashlyn cerniglia Students also viewed Exam 4 27 terms beverly345 Preview Chapter 4 Chemical Bonds 93 terms

https://quizlet.com/362439002/chemistry-chapter-8-covalent-bonding-flash-cards/
23 terms Sinclairjmn Preview 8 1 Molecular Compounds 25 terms jmorg0605 Preview Terms in this set 48 Section 8 1 Section 8 1 Covalent bond Bond formed by the SHARING of electrons between atoms Molecule A neutral group of atoms joined together by covalent bonds Diatomic molecule a molecule that contains two atoms
https://quizlet.com/106914846/study-guide-covalent-bonding-chapter-8-flash-cards/
A way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by one single Lewis formula covalent bonds How does VSEPR theory help predict the shape of molecules predicts that the electron pairs will repel and the shape of the molecule will reflect

https://quizlet.com/570840108/chapter-8-covalent-bonding-test-study-guide-flash-cards/
Created by Rowan1061 Terms in this set 46 When sharing of electrons occurs the attachment between atoms that results is called a covalent bond When such an attachment is formed bond dissociation energy is released and the process is exothermic
Similarities Both types of bonds result from overlap of atomic orbitals on adjacent atoms and contain a maximum of two electrons Differences bonds are stronger and result from end to end overlap and all single bonds are bonds bonds between the same two atoms are weaker because they result from side by side overlap and multiple bonds contain one or more bonds in addition to a Chapter 8 study guide covalent bond 57 cards Chemistry Chemistry when covalent bonds are formed bond dissociation energy is released and the process is exothermic greater amount of energy is required to break a bond in reactants than is released when the new bonds form in the products
Ch 8 Covalent Bonding Name Ch 8 Covalent Bonding Before You Read Review Vocabulary ionic bond Define the following terms octet rule Chapter 4 Describe the structure of an atom Chapter 6 Explain the following concepts periodic trends and periodic properties of elements Chapter 8