Chapter 8 Chemistry Study Guide Covalent bond in a covelant bond the dissociation energy is released in the process of exothermic reaction When 2 or more atoms bond by means of electron sharing the result is a molecule shared electrons are centered between the 2 atoms in covalent bonding the attachment is called a sigma bond
Most elements found in nature with the exception of the exist as molecules noble gases a neutral group of atoms joined together by covalent bonds molecule Two properties of molecular compounds 1 Low melting and boiling points 2 gases and liquids Shows the number of atoms of each element in a molecule Step by step solution Step 1 of 1 Mendeleev s periodic law helped in classifying elements into groups with similar properties Mendeleev s left certain vacant places in his table which provided a clue for discovery of new elements The atomic masses of certain elements were corrected using Mendeleev s periodic law
Chapter 8 Chemistry Study Guide
 Chapter 8 Chemistry Study Guide
Chapter 8 Chemistry Study Guide
https://s3.studylib.net/store/data/025387342_1-9dc53cb57a03ded1e738f5eeda3ab83e.png
Chemistry It takes 8 17 times 10 19 mathrm J of energy to remove one electron from a gold surface What is the maximum wavelength of light capable of causing this effect
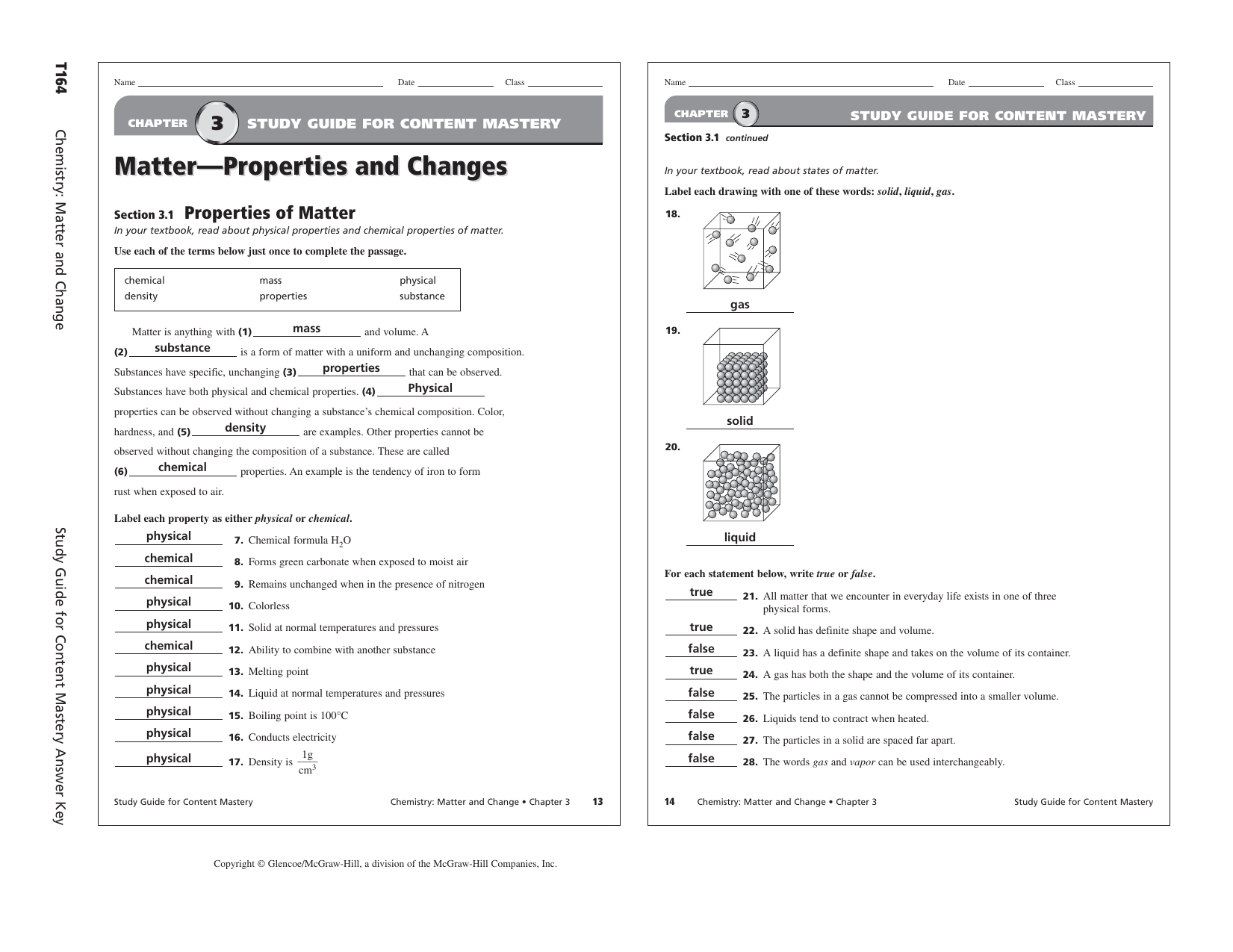
Pre-crafted templates use a time-saving solution for producing a diverse range of documents and files. These pre-designed formats and designs can be utilized for numerous individual and professional projects, consisting of resumes, invitations, flyers, newsletters, reports, presentations, and more, streamlining the content development process.
Chapter 8 Chemistry Study Guide

Chemistry Second Semester Final Exam Study Guide Answers Study Poster

CHAPTER 8 REVIEW
Chemistry Study Guide

CHEMISTRY STUDY GUIDE IB PDF

2nd Year Chemistry Chapter 8 Solved Exercise Exercise Poster

Download PDF EPUB And Mobi Ebooks Download Study Guide And Solutions
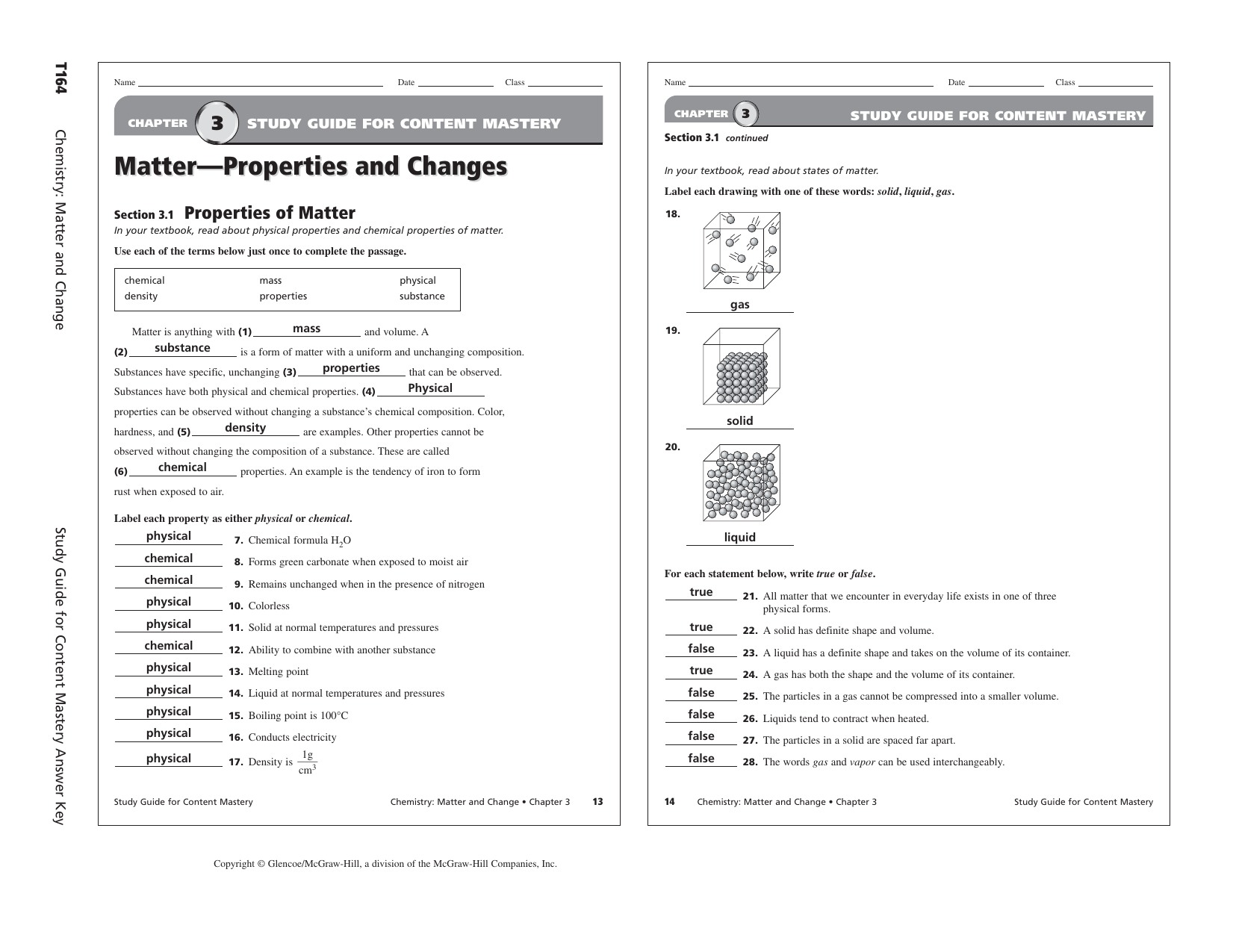
http://www.mchsapchemistry.com/uploads/7/7/1/5/7715124/chapter_8_study_guide.pdf
Accelerated Chemistry Anderson MCHS Modern Chemistry 7 Chemical Equations Reactions CHAPTER 8 STUDY GUIDE Chemical Equations and Reactions SECTION 8 3 SHORT ANSWER Answer the following questions in the space provided 1 List four metals that will not replace hydrogen in an acid

https://quizlet.com/81215117/chemistry-study-guide-chapter-8-flash-cards/
Rule 2 ion rule The oxidation number of a monatomic ion is equal to the charge of the ion eg When Br atom gains an electron to become Br ion it has an oxidation number of 1 When Mg atom loses two electrons to become Mg2 its oxidaton number becomes 2 Rule 3 the zero sum rule The sum of the oxidation numbers of all the atoms in a

https://openstax.org/books/chemistry/pages/chapter-8
Similarities Both types of bonds result from overlap of atomic orbitals on adjacent atoms and contain a maximum of two electrons Differences bonds are stronger and result from end to end overlap and all single bonds are bonds bonds between the same two atoms are weaker because they result from side by side overlap and multiple bonds contain one or more bonds in addition to a

https://quizlet.com/554044946/chemistry-chapter-8-study-guide-flash-cards/
Solution A solution B and the mixture are all yellow In the reaction described by the word equation sodium water sodium hydroxide hydrogen gas Both a and c Which of the following indicates that a chemical equation is balanced The numbers of atoms of each element are the same on both sides of the equation

https://quizlet.com/125242675/chapter-8-chemistry-study-guide-flash-cards/
Electron sharing occurs so that atoms attain the electron configuration of noble gases Single Covalent Bonds Two atoms held together by a sharing of a pair of electrons are joined by a single covalent bond Electron Dot Structure represents the shared pair of electrons of the covalent bond by two dots Structural Formula
General Chemistry I Chapter 8 Professor Deak Course General Chemistry I CHEM101101 47 Documents Students shared 47 documents in this course University Boston College Info More info Academic year 2020 2021 Uploaded by Anonymous Student Chapter 8 Study Guide The gas behavior most like an ideal gas will occur under the conditions that minimize the chances of significant interactions between the gaseous atoms molecules namely low pressures fewer atoms molecules per unit volume and high temperatures greater kinetic energies of atoms molecules make them less susceptible to attractive forces
A amount of air remaining in the lungs after quiet expiration ERV RV B Measure of the strength of respiration RV IRV ERV C total ability to inspire TV IRV D total amount of air that can be in the lungs E volume of additional inspired air above and beyond the tidal volume F volume of air left in the lungs following forced