Chapter 6 Study Guide Chemistry Terms in this set 161 Chemical Bonding The force of attraction that holds 2 atoms together the interferance of valence electrons and all atoms bond to decrease potential energy Ionic bonding The transfer of electrons non metals takes in electrons to make negative ions metals to non metals and also called electrostatic attraction Metal
Study with Quizlet and memorize flashcards containing terms like A chemical bond results from the mutual attraction of the nuclei of atoms and a electrons b neutrons c protons d dipoles As independent particles atoms are a at relatively high potential energy b very stable c at relatively low potential energy d part of a chemical bond As atoms bond with each other they 44 Chemistry Matter and Change Chapter 6 Study Guide Section 6 2 Classification of the Elements In your textbook read about organizing the elements by electron configuration Use the periodic table on pages 178 179 in your textbook to match each element in Column A with the element in Column B that has the most similar chemical properties
Chapter 6 Study Guide Chemistry
Chapter 6 Study Guide Chemistry
http://chemistrygeek.com/study guides/Chemistry I HD/Chem I-1 HD Final Study Guide Answers 2012 p1.JPG
Chemistry Chapter 6 Study Guide STUDY Flashcards Learn Write Spell Test PLAY Match Gravity Created by alligraz The Periodic table and Periodic law Terms in this set 35 Actinide Series the periodic table the f block elements from period 7 that follow the element actinum Alkali Metal
Templates are pre-designed documents or files that can be used for numerous purposes. They can conserve time and effort by offering a ready-made format and layout for producing different kinds of material. Templates can be utilized for personal or professional projects, such as resumes, invites, leaflets, newsletters, reports, presentations, and more.
Chapter 6 Study Guide Chemistry

Pin On Chemistry

General Chemistry Chapter 4 Homework
Study Guide Answers
Chapter 6 The Periodic Table Worksheet Answers Pearson Brokeasshome

Study Guide for Chemical Principles 6th edition PDF E book

Functional Groups Part 1 Organic Chemistry chemistry organic
http://www.mchsapchemistry.com/uploads/7/7/1/5/7715124/ch_6_chemical_bonds_-_obj__study_guide.pdf
CHAPTER 6 STUDY GUIDE Chemical Bonding SECTION 1 INTRODUCTION TO CHEMICAL BONDING Modern Chemistry 6 Chemical Bonding SECTION 1 continued 10 If electrons involved in bonding spend most of the time closer to one atom rather than the other the bond is 11 If a bond s character is more than 50 ionic then the bond is called a n

https://www.livingston.org/cms/lib4/NJ01000562/Centricity/Domain/794/Chapter%206%20study%20guide%20answer%20key.pdf
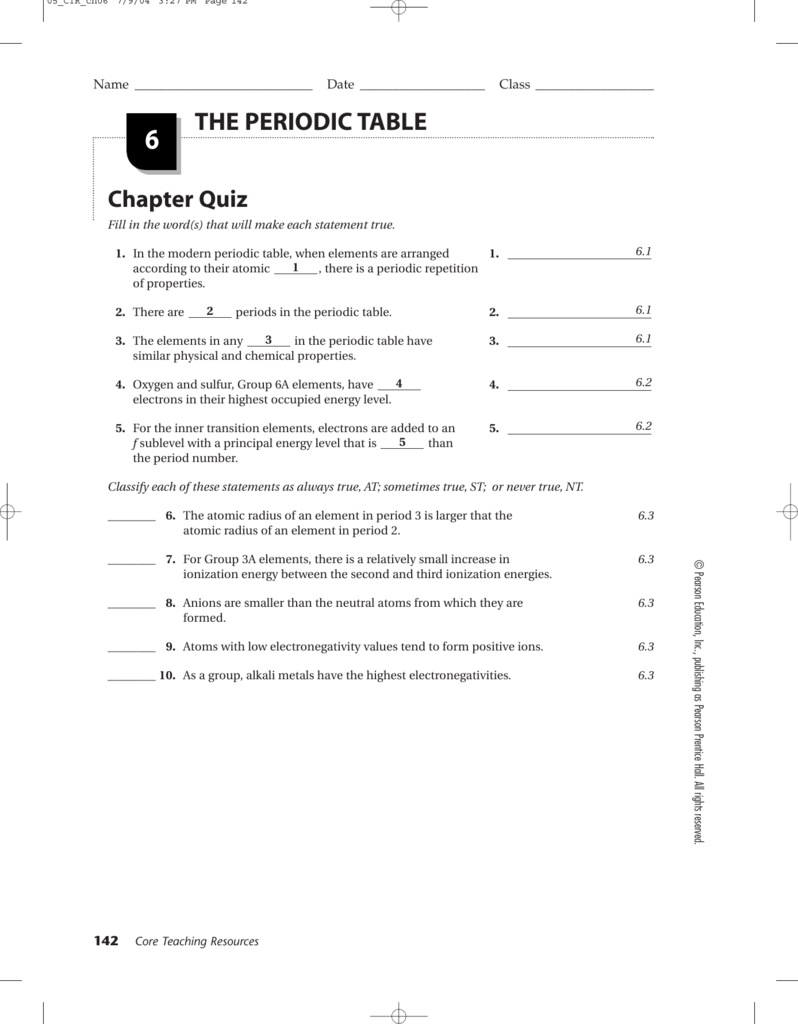
CHAPTER Date STUDY GUIDE FOR Class CONTENT MASTERY Name CHAPTER Section 6 2 continued Date Class STUDY GUIDE FOR CONTENT MASTERY Section 6 2 Classification of the Elements In your textbook read about organizing the elements by electron configuration Use the periodic table on pages 156 157 in your textbook to match each element in

https://quizlet.com/170448353/chemistry-chapter-6-study-guide-flash-cards/
The bar graph shows the relationship between atomic and ionic radii for Group 1A elements a Describe and explain the trend in atomic radius within the group b Explain the difference between the size of the atoms and the size of the ions A The atomic radius increases in a group with the increasing atomic number

https://quizlet.com/17183693/chemistry-chapter-6-study-guide-flash-cards/
A theory that predicts some molecular shapes by using the idea that pairs of valence electrons surrounding an atom repel each other is called the VESPR theory is short for valence shell electron pair theory chem study guide chapter 6 12 5 12 Learn with flashcards games and more for free

https://quizlet.com/373006369/chemistry-chapter-6-mcgraw-hill-flash-cards/
Study with Quizlet and memorize flashcards containing terms like Periodic law representative elements transition elements and more McGraw Hill Chemistry Chapter 6 18 terms fl3tching101 Preview Chemistry Chapter 6 Study Guide 35 terms alligraz Preview Unit 5 Chemistry Periodic table periodic trends 17 terms ryan andrew714
84 Study Guide for An Introduction to Chemistry Section Goals and Introductions As always the Review Skills section for this chapter is important A quick review of atomic mass and moles in Section 3 6 the information on ionic and molecular compounds in Section Chapter Test Practice Careers in Chemistry Concepts in Motion Interactive Tutor Personal Tutor Vocabulary eFlashcards Section 1 Development of the Modern Periodic Table Section 2 Classification of the Elements Section 3 Periodic Trends
13 1 Study Guide for Chapters 6 and 7 Certain topics like the structure synthesis and nomenclature of alkyl halides have already been covered previously This part of the course is primarily concerned with Sn1 and Sn2 reactions E1 and E2 reactions and alkene synthesis structure and nomenclature The following is the suggested order of