Acids And Bases Study Guide Goals To describe acids To make the distinction between strong and weak acids To show the changes that take place on the particle level when acids dissolve in water To show how you can recognize strong and weak acids This section introduces one way to define acids called the Arrhenius definition
Sulfuric acid is the strongest acid on our list with a pK a value of 10 so HSO 4 is the weakest conjugate base You can see that hydroxide ion is a stronger base than ammonia NH 3 because ammonium NH 4 pK a 9 2 is a stronger acid than water pK a 14 00 The stronger the conjugate acid the weaker the conjugate base Compare the acid base models of Arrhenius Br nsted Lowry and Lewis and know the salient features of each ISBN 9783527304134 See also the study guide and other publisher resources Brandis Kerry Acid Base Physiology See Chapter 1 of this online tutorial textbook which applies acid base chemistry to physiology People Antoine
Acids And Bases Study Guide
 Acids And Bases Study Guide
Acids And Bases Study Guide
http://msbeaucage.weebly.com/uploads/1/2/7/5/12751975/wks4_1p1.key.gif
Meanwhile the Br nsted Lowry definition of acids and bases defines acids and bases in the form of a donation reaction Basically an acid base is seen as an H donator or accepter the acid donates the base accepts HA B HB A In this example HA is the acid which donates an H ion to the B ion the base to form HB and A
Templates are pre-designed files or files that can be used for various functions. They can save time and effort by offering a ready-made format and layout for creating different sort of content. Templates can be used for personal or professional tasks, such as resumes, invites, flyers, newsletters, reports, discussions, and more.
Acids And Bases Study Guide

Acid Base Reactions Quiz MM s Website

Science Acids And Bases Study Guide

Unit IX Study Guide Acids And Bases Chapters 14 15 And 18 3 To

Chemical Reactions Study Guide

Solutions Acids Bases Study Guide
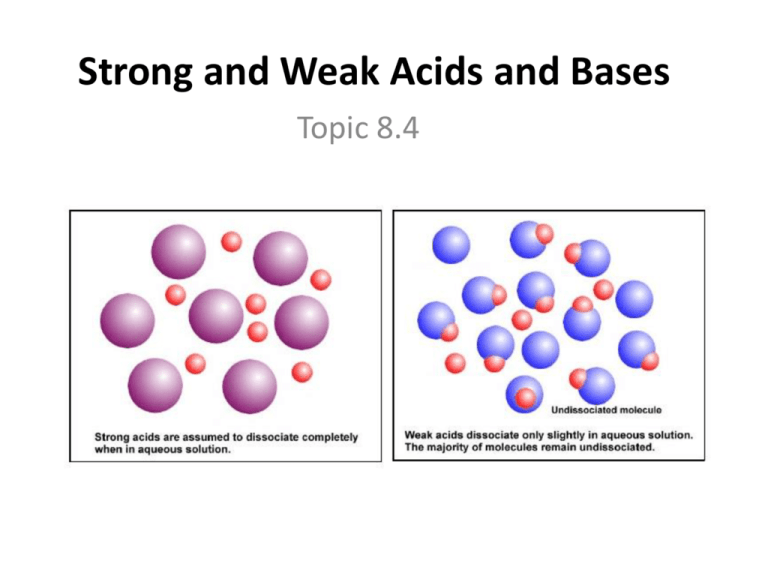
Strong And Weak Acids And Bases

https://chem.libretexts.org/Bookshelves/General_Chemistry/Chem1_(Lower)/10%3A_Fundamentals_of_Acids_and_Bases/10.01%3A_Introduction_to_Acids_and_Bases
Arrhenius Definition an acidic substance is one whose molecular unit contains at least one hydrogen atom that can dissociate or ionize when dissolved in water producing a hydrated hydrogen ion and an anion hydrochloric acid HCl H aq Cl aq sulfuric acid H 2 SO 4 H aq HSO 4 aq

https://www.khanacademy.org/science/chemistry/acids-and-bases-topic
Learn Arrhenius acids and bases Arrhenius acids and bases pH pOH and the pH scale Br nsted Lowry acids and bases Br nsted Lowry acids and bases Autoionization of water Water autoionization and Kw Definition of pH Strong acid solutions Strong base solutions
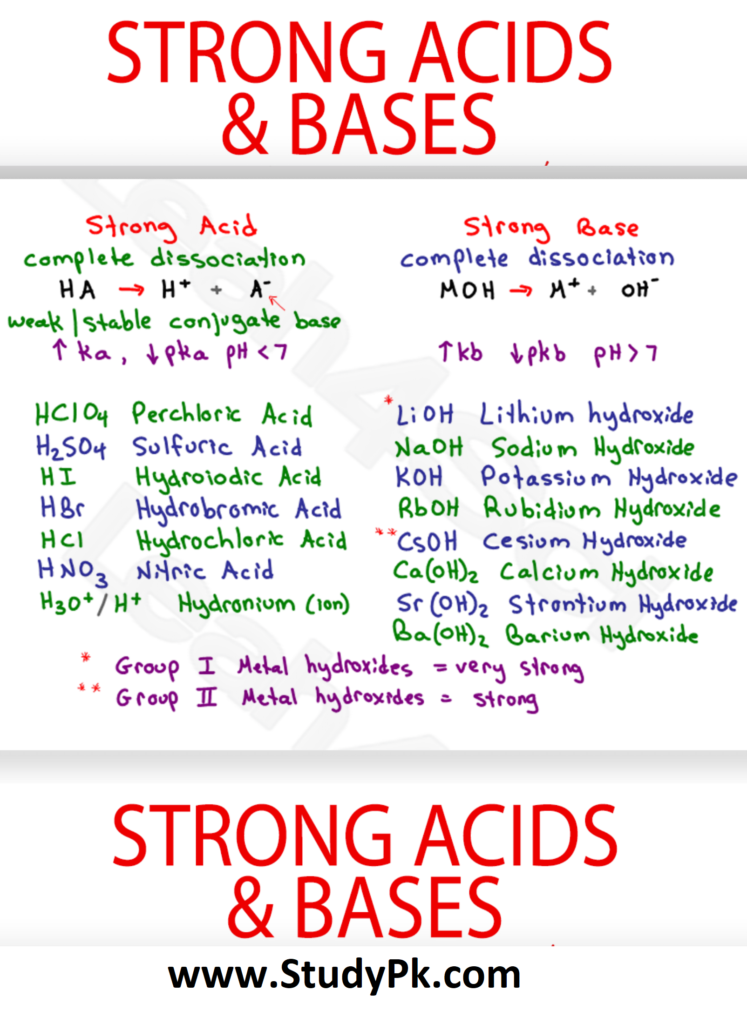
https://chem.libretexts.org/Courses/Thompson_Rivers_University/TRU%3A_Fundamentals_and_Principles_of_Chemistry_(CHEM_1510_and_CHEM_1520)/06%3A_Acid-Base_Equilibrium/6.S%3A_AcidBase_Equilibrium_(Study_Guide)
Br nsted Lowry definition of acids an bases Acid is a proton donor Base is a proton acceptor Can be applied to non aqueous solutions Br nsted Lowry acid must be able to lose a H ion Br nsted Lowry base must have at least one non bonding pair lone pair of electrons to bind to H ion Amphoteric substance that can act as an acid or

https://chem.libretexts.org/Courses/University_of_California_Santa_Cruz/UCSC%3A_Chem_1B-AL_(Mednick)/Worksheets%3A_General_Chemistry/Introduction_to_Acids_and_Bases_(Worksheet)
Br nsted Lowry The Br nsted Lowry definition of acids and bases liberates the acid base concept from its limitation to aqueous solutions as well as the requirement that bases contain the hydroxyl group A Br nsted Lowry acid is a hydrogen containing species which is capable of acting as a proton hydrogen ion donor A Br nsted Lowry base is a species which is capable of acting
https://chem.libretexts.org/Bookshelves/General_Chemistry/Chem1_(Lower)/10%3A_Fundamentals_of_Acids_and_Bases
10 1 Introduction to Acids and Bases The concepts of an acid a base and a salt are very old ones that have undergone several major refinements as chemical science has evolved Our treatment of the subject at this stage will be mainly conceptual and qualitative emphasizing the definitions and fundamental ideas associated with acids and bases
In chemistry an acid is a substance that has a pH of less than 7 0 when dissolved in water Acids have a sour taste like the acidity of a lemon and can react with bases to form salts They also have the ability to donate protons H to other substances which is why they are also called proton donors Base Acid Curved arrow mechanism ofacid basereactions Startthearrow fromthelone pair negative charge ofthebase andattackthemostacidic hydrogen oftheacid Break thebondofthehydrogen andmove theelectrons totheotheratom Remember curved arrowsshowmovement ofelectrons BothAhhrenius andBr nsteddefinitions arefromProtonProspective
Unit 3 Properties and Definitions of Acids and Bases References and Notes Work to Submit Study Guide Page 6 Chemistry 3102B Study carefully the Sample Problems Conjugate Acid Base Pairs and More Conjugate Acid Base Pairs pages 555 556 Then answer question 3 8 and 3 9 See your instructor to find out which questions you should